Transmission
Stories from the inside
Transmission is the award-winning podcast of the Institute of Tropical Medicine in Antwerp. Our enlightening and intimate series shares the personal and professional experiences of researchers and physicians as they relentlessly battle diseases.
Listen now

Outbreaks and emerging infectious diseases
Season 1
Welcome to Transmission, an immersive journey into the heart of global health battles, featuring the researchers at, and partners of, the Institute of Tropical Medicine in Antwerp. From early encounters with Ebola in distant villages to the recent fight against COVID-19 in bustling urban settings: these are stories of resilience, discovery and humanity in the most challenging circumstances imaginable.
And so it begins...
Transmission #1
Listen to real-life stories from the frontlines of the fight against Ebola and other deadly infectious diseases. Get ready to learn more about where outbreaks start and why, and how viruses evolve, leaping from animals to humans.
And so it begins...
Transmission #1
Kevin Ariën: If, if there is really a new pathogen - a new virus - we will not see it coming.
Narrator: This is Transmission, the podcast of the Institute of Tropical Medicine in Antwerp. In this podcast, we will uncover the mysteries of diseases that impact us all and delve into the cutting edge science of keeping people healthy. We invite you to look over the shoulders of the experts who make it their life's mission to improve global health. In this first episode of Transmission, you will find out where outbreaks start and why, and how viruses evolve. Transmission, your front row seat to the world of health, science and beyond.
Narrator: On Wednesday, the 29th of September 1976 a special package was delivered to ITM, the Institute of Tropical Medicine in Antwerp. It was a cheap thermos flask made of glossy blue plastic. The thermos contained two test tubes filled with congealed blood from a sick Flemish nun living in the Democratic Republic of the Congo, which was called Zaïre at the time. One of the test tubes was broken, so some blood got mixed with the melted ice at the bottom of the flask. Peter Piot, a young researcher who had just started working at the lab, and two of his colleagues set out to examine the blood. Little did they know that they were handling one of the most dangerous substances on earth without any protection. One day later, on September the 30th, the nun died in Zaïre. But she was not the only one. The mysterious disease also killed several local residents. Peter Piot and his team were urgently called to investigate the case on site. Not easy, because due to the outbreak of the disease, the region was in lockdown and no one could get in or out. Nonetheless, the ITM team traveled to Zaïre and tried to find transportation that would get them to the lockdown area.
Narrator: But first we need to back up a little. The story of our mysterious disease actually starts earlier. Six days before the researchers at ITM received the blue thermos, Jean-Jacques Muyembe, a Congolese microbiologist, arrived at an empty hospital in Yambuku in Zaïre.
Jean-Jacques Muyembe: This was a terrible disease, that time we called ‘mysterious disease’ with a lot of, euh, deaths in the population and also among the nurses. The minister of health sent me to Yambuku, that was euh, in euh, 23 September 1976. And when I arrived there in Yambuku, the hospital was empty because the population fled the hospital saying that euh, this is the place of death.
Narrator: Muyembe quickly finds the only two other residents of the hospital, a mother and her child. Not much later, the child dies, the mother leaves the hospital. The place is completely empty now. Muyembe goes to sleep.
Narrator: The next morning as people hear Muyembe has been sent by the central government, they start to line up at the hospital hoping he has medicine for them. Muyembe starts doing what he is trained for: collecting samples and investigating the disease. He also has to draw blood.
Jean-Jacques Muyembe: At that time, I didn’t think that it was euh, a dangerous or an extraordinary euh, disease. Because the disease was like euh, malaria, like typhoid fever.
Narrator: Muyembe looks for gloves, but can’t find any on the hospital premises, so he starts working without them. The syringe to take blood plunges into the arm of a patient. But when he removes the syringe, something unexpected happens: the puncture doesn’t close, it starts gushing blood.
Jean-Jacques Muyembe: My finger was euh, soiled with the, with the blood of the patients. So immediately I asked soap and water to, to wash my hands, otherwise I would not be here with you. (laughter) Yes, yes I survived, I was lucky at that time. So this, this was my first encounter with euh, this euh, this virus.
Narrator: Doctor Muyembe goes on to investigate the disease to see which action he must take.
Jean-Jacques Muyembe: At the lunch, we were invited by the sisters, the nuns there from Belgium, so euh, they told me that euh, one of the nuns was euh, ill. And she was working also in the hospital. So we concluded that euh, the diseases were severe there, euh, we must go back to Kinshasa to accelerate the diagnosis of euh, this illness. So we decided to, to leave the mission and euh, we took with us these euh, Belgian nuns euhm, and we euh, traveled to Kinshasa.
Narrator: He then takes blood samples, but not much later…
Jean-Jacques Muyembe: The situation of euh, of the sister we bring to Kinshasa, euh, started to worse, and it is why we decided to take blood and send to ITM here, euh, where euh, professor Piot was working.
Narrator: Realizing he was dealing with a mysterious and deadly disease, he puts the blood samples into a glossy blue thermos that he sends to the Institute of Tropical Medicine in Belgium for further investigation. Here, Peter and his colleagues open it up, and examine its contents…
Jean-Jacques Muyembe: They find that euhm, the disease was caused by a virus.
Narrator: Peter and his colleagues also see the danger of the disease and rush to Zaïre and to Jean-Jacques Muyembe to support the search for the source of this unknown outbreak.
Narrator: The pilots don’t like to have to go to the quarantined area to drop off a bunch of researchers. After they have unloaded the last box, they shout, ‘bonne chance’ and fly off. Jean-Jacques Muyembe, Peter Piot and the rest of the team remain behind to search for the source of this unknown outbreak.
Jean-Jacques Muyembe: Later on, this virus will be euh, called Ebola, Ebola virus. So this is the, the long history of euh, of the disease, yes.
Narrator: Ebola is one of the deadliest diseases we know. The outbreak in 1976 would eventually kill 280 people in Zaïre. But it wasn’t the last one. Since its discovery there were several big Ebola outbreaks with the biggest one in 2014 causing more than 11,000 reported deaths. But Ebola isn’t a stand-alone case. Infectious diseases such as covid, dengue, zika and different kinds of flu seem to be appearing more and more aggressively throughout the world. Researchers globally are discovering new infectious diseases at an unprecedented rate. And more and more, these diseases push against the limits of our health systems, threatening to become the next global pandemic.
Kevin Ariën: I don’t believe we will, we will have a pandemic at some point in time that, that kills the last man on earth.
Narrator: This is Kevin Ariën, virologist at ITM. But will there ever be a new pandemic that kills millions of people?
Kevin Ariën: Yes. That is a guarantee.
Narrator: And that disease will probably come somewhere out of the jungle. It already exists there, and more and more people are invading its territory.
Narrator: We are flying high above the Congolese rainforest, a jungle about 2 million square kilometers in size - an endless sea of trees. It spans six different countries. This mind-blowing place offers a safe home to thousands of species of trees, plants and animals. From elephants to gorillas but also birds, fish, insects, snakes and rodents. It’s a hot brooding sea of green that stretches endlessly when viewed from above.
Narrator: We are underneath the canopy now. The light changes. The trees filter the sunlight. It is full of insects. An elephant would be difficult to find. Here and there, you hear squeaking and the rustling of leaves. You hear a droplet falling from a leaf. A leftover from the last storm. But then in the distance, you hear…. Joseph and his friends left their village early this morning and have been on the road for several hours now. In the Congolese rainforest there is industrial forestry, but wood is also felled for daily use. Joseph knows the forest very well, he is an expert in how to survive here. He knows where to put his feet and what animals to look out for when he pauses near a river. He knows the plants he can eat and those he should really stay away from. But despite the fact that he knows this part of the forest like the back of his hand, what he doesn’t realize is that he is in a killer’s territory. A killer he can’t see or feel. It isn’t a predator that eats him from the outside but a virus that will attack him unknowingly from the inside. It’s a virus that floats around in the body of a jungle rat or bird but will soon make the jump from its normal host to Joseph’s body. Of course, the virus doesn’t attack consciously; it doesn’t wait in the branches of a tree ready to jump onto a passing lumberjack. No, a virus is sneakier than that. It relies on casual contact. We don’t know how this transmission will happen. It could be a bat that Joseph startles in a cave, the fleas of a rat he’ll chase away or swift contact between a small scratch on his hand and the blood of an animal he shot on his way home to cook. How it happens, we don’t know. But it is certain that somewhere, somehow a virus will see its chance to jump from the animal to Joseph’s body.
Narrator: Such transmissions from animal to human are not rare at all.
Kevin Ariën: This probably happens multiple times per day on this world.
Narrator: This is Kevin Ariën again. Luckily for us, a virus that is transmitted to humans typically cannot do anything inside the human body. It is a strange environment that the virus doesn’t know and in which it cannot reproduce. In 99% of the cases, we don’t even know that we’re infected. But…
Kevin Ariën: In very exceptional cases that person could die.
Narrator: And if we are really unlucky, the virus feels at home in our bodies, and is not only transmitted from animal to human, but also from human to human. And then we’re in for one hell of a ride.
Narrator: Ebola is a virus that is regularly transmitted from animals to humans. The Spanish flu which killed about 30 to 50 million people in the beginning of the 20th century also took the jump from wild birds. There are many more viruses that decided to make the leap and enter our bodies. Like the regular annual flu virus. But also Covid, Mpox, lassa fever, Marburg, zika and many, many more.
Kevin Ariën: It’s like an experiment of nature. Viruses are, are extremely diverse and, and they come in, in different flavors, euhm, nature has tried out every possible thing with viruses and that is still seen today in the diversity that, that viruses represent.
Narrator: Viruses often look like a kind of ball with spikes or in the case of Ebola, more like a walking stick.
Kevin Ariën: This is really close to the perfect pathogen.
Narrator: They are constructed very simply out of a bit of genetic material with a smidge of protein and a thin layer of fat to encapsulate both. That’s it. With this simple built they can inject their genetic material into us and make copies of themselves using our own cell infrastructure.
Kevin Ariën: That’s what fascinates me, that’s the beauty of viruses - the fact that they are so simple yet can have such yeah, profound effects on their hosts.
Narrator: Viruses evolve quickly. And unlike bacteria you cannot treat them with antibiotics. They are extremely diverse and can be deadly. From Mers to SARS and from yellow fever to swine flu. And on top of that, we don’t have a good understanding of how viruses do what they do. Even the ones that have been circulating in humans for a long time. Kevin for example studies Dengue at the ITM lab in Belgium. Here, they have a high security biosafety level 3 lab. Specifically designed for working on dangerous pathogens.
Kevin Ariën: People die of Dengue every year. And it’s not well understood euhm, why that is, the pathogenic mechanism of that virus is not well understood.
Narrator: And we don’t even know how the Dengue virus makes people sick. A virus we’ve already known for years, so it’s even more difficult to understand diseases we rarely come into contact with.
Kevin Ariën: For many of those viruses, tests are not yet available or will not be available.
Narrator: Most new and unknown viruses hide inside the animals in the forest and most of them stay hidden there too. They multiply in the cells of a rat, a bat, a monkey or a bird. They usually do not kill the animal but live a happy life far away from humans.
Kevin Ariën: There is a lot of, of animals in, in the wild that carry viruses.
Narrator: But they can only be transmitted to humans once we get into contact with those animals. When we go into the woods and start shaking the trees, we shouldn’t be surprised that things fall out.
Laurens Liesenborghs: The virus for the next pandemic is already circulating in one of these animal species.
Narrator: This is Laurens Liesenborghs, infectious disease expert at ITM.
Laurens Liesenborghs: If we enter and we disturb their habitats and increase our exposure to these animals, euh, of course there will be much, much more spillover events.
Narrator: When we shake a virus out of its regular host, by killing the animal host, or by destroying its habitat, the virus has two options. It has to find a new host, a new kind of host or go extinct. It’s not that viruses target humans especially, but if you look at the world from the point of view of a virus, we offer a magnificent feeding ground and a marvelous target with all our billions of human bodies. And the more habitats we destroy, the more animals have to move around and the more interaction there is between us humans and those animals. We cut our way through the forest of Congo. We cut roads through the Amazon, through the forests of Borneo, Madagascar, New Guinea or Cambodia. We build villages very close to the edge of the forest and we eat the meat of animals we catch there.
Kathy Kreppel: And yeah, that will give us more problems.
Narrator: This is epidemiologist Kathy Kreppel.
Kathy Kreppel: More pandemics and new pathogens that are totally different families than what we’ve dealt with before. In Indonesia now you get certain monkeys coming closer to the people working in the palm oil plantations.
Narrator: There are mosquito species that used to only sting monkeys and other animals in the forest. But due to the increasing interaction with humans a new malaria parasite has evolved inside these mosquitos.
Kathy Kreppel: Its simple constant, the mosquitos just had monkey and people available at all times. And then the parasite, just one of them, had a little bit of a DNA change and suddenly it could replicate in a human.
Narrator: Nature has to adapt to humans and infectious diseases just follow along. But it doesn’t always have to happen in the forest either. We eat meat from densely packed industrial farms where we push animals as closely together as possible, creating the ideal circumstances in which viruses and parasites can multiply in the animals we later eat. And also we humans start to live closer to each other.
Kevin Ariën: By 2050 it is expected that we hit 10 billion people globally and that up to 70% of that global population, so 7 billion people will be living in, in cities. And the, the biggest cities already today, but also in 2050 are found in Southeast Asia.
Narrator: So a lot of people are living close together in Southeast Asia, a region where a main source of fresh food are wet markets. Places where you can get live animals that are sometimes slaughtered at the market. That makes those wet markets places with an intense contact between a lot of people and a lot of animals. Diseases such as SARS, MERS or Covid all originated in Southeast Asia.
Laurens Liesenborghs: So those, those circumstances will only increase, will only become better in the years to come.
Narrator: Combine those factors with the rising international plane travel and some day in the not-too-distant future, a new infectious disease will make the leap from animal to human again. That disease will feel at home in our bodies and eventually be transmitted from human to human. And cause the next global pandemic.
Narrator: If we know that infectious diseases are a big problem and if we know that a pandemic is coming anyway, can’t we prevent it? Or at least prepare for the next outbreak? Every infectious disease has its own riddles to solve. In which animal is it located, how is it transmitted from person to person and how do people deal with the disease? We need a team to keep watch and at the same time tackle the intricate enigmas of every possible disease. And that team exists. They gather people from all around the world, from Asia to Africa, they keep a watchful eye. They guard the boundaries between humans and infectious diseases. How are they solving the complex problems that the disease puts on their path? That is what we’ll find out in the next episodes. We’ll get stuck in the mud on our way to remote villages, pitch our tent under a tree full of snakes and see entire laboratories descend from the sky as we follow experts around the globe in their quest to understand the world of infectious diseases.
Johan Van Griensven: I think, yeah, the most stressful moment, period of my life, I wouldn’t recommend it to my enemy.
Narrator: Thanks for listening. Join us next time when we unravel the complex riddles pathogens throw on our path. For more information on the Institute of Tropical Medicine in Antwerp, please go to ITG.be/podcast.
Solving the riddle
Transmission #2
Meet the members of the Outbreak Research Team. Follow in the footsteps of the most talented scientists as they travel the world to get a grasp on global health challenges, or toil away in hi-tech labs to solve the complex riddles that come with new infectious diseases.
Solving the riddle
Transmission #2
Laurens Liesenborghs: If something goes wrong, it can go wrong quickly and, and very badly.
Narrator: This is Transmission, the podcast of the Institute of Tropical Medicine in Antwerp. In this podcast, we will uncover the mysteries of diseases that impact us all and delve into the cutting edge science of keeping people healthy. We invite you to look over the shoulders of the experts who make it their life's mission to improve global health. In our first episode, we met professor Jean-Jacques Muyembe, one of the scientists who first discovered Ebola and researched how this infectious disease was being transmitted. In this second episode of Transmission, we delve deeper into the complex riddles pathogens throw on our path and the intriguing research that is being done to stop them. Transmission, your front row seat to the world of health, science and beyond.
Narrator: Laurens Liesenborghs and Placide Mbala-Kingebeni are experts in infectious diseases. They travel the world to unravel the mystery of monkeypox or Mpox as it is officially called. They go far and wide always on the move but right now their four-by-four is stuck in the mud.
Laurens Liesenborghs: Yeah well, the Congolese have a very nice word in French, it is ‘embourbé’. The word means when a, when a vehicle gets stuck in the mud euh, literally, euh, which is, one of the most euh, often used words that you experience on such a trip.
Narrator: The car is leaning precariously, and everyone is trying to get the vehicle back on track. It is 2022, we are in the DRC, short for the Democratic Republic of the Congo. And we are on the road to a remote village in the rainforest. But as Laurens explains…
Laurens Liesenborghs: Yeah, unfortunately, once you get out of the capital the roads become really, really bad.
Narrator: And when it rains, everything floods.
Narrator: Placide and Laurens can finally get back in the car. But there is still a long way to go.
Laurens Liesenborghs: It’s a real hassle to get anywhere, especially in rainy seasons. They are completely cut off from the outside world and they are completely self sufficient as well.
Narrator: Laurens, Placide and the rest of the team are researching an outbreak of Mpox. A disease that starts with small blisters that often spread all over the body. After a while, these blisters become large bumps filled with pus. Overtime they disappear but they leave large scars and wounds that often inflame.
Laurens Liesenborghs: Often they also go to the eyes, so we saw a lot of people with blindness as a consequence of an Mpox infection.
Narrator: In the DRC, between 1 and 10% of Mpox patients die. And this is why Laurens and his colleagues go to the hotbed of the disease to study it.
Laurens Liesenborghs: It’s a disease that we find really in the heart of the rainforest in, in the Democratic Republic of the Congo.
Narrator: Laurens and Placide were studying one of the largest outbreaks ever recorded in the country. And getting to the heart of the rainforest was not easy. First they had to take internal flights, then they drove two days by car. When that became impossible, they transferred to a motorcycle. And of course also…
Laurens Liesenborghs: Wading through the rivers and so we were waist high in the water searching for these Mpox cases.
Narrator: After all those challenges and setbacks they arrived at the village. Laurens took time to talk to the villagers, get to know how they live with the disease and get a better understanding of the whole spectrum of Mpox transmission. From animal to human and from human to human.
Laurens Liesenborghs: We returned to the provincial capital where finally we had some internet connection.
Narrator: But when they opened their computers…
Spreker: Er is een eerste geval van het apenpokkenvirus opgedoken in…
Translation: A first case of Mpox has surfaced in Belgium, so the virus has also reached our country. And we have a few questions for Isabel Brosius of the Institute of Tropical Medicine in Antwerp: you and your team have been researching the Mpox virus?
Isabel: Ja, dat klopt, ….
Translation: Euh, yeah, that’s right. The patient presented himself at the Institute of Tropical Medicine here in Antwerp. With symptoms for which the necessary samples were taken to be able to confirm the infection.
Laurens Liesenborghs: All of a sudden we found out that there, hey, there’s already been five cases of Mpox at the Institute of Tropical Medicine in Antwerp.
Narrator: The variant of the virus that spread in Europe was much less dangerous. Still, infectious disease specialist Isabel Brosius found herself at the center of what was happening in Belgium.
Isabel Brosius: I work very closely with euh, with Laurens and I, normally would have gone together on this euhm, field trip euh, but I, I was pregnant at the time, so I, I was forced a bit to observe from the side but then while they were there, I, I mean, how big the coincidence can it be, there is a certain moment where, yeah, we had the news via all sorts of scientific networks of cases om Mpox that were suddenly euh, being reported, UK, some in the US, some in Spain, euhm, so already we had the sense like, okay, this is out of the ordinary, euh, we should start preparing and then only two days afterwards, euh, we had our first case. There was this whole media storm that actually broke loose a bit and, and at that time, I, I was, well one of the few people then still at ITM that was actively involved already in, in, in research on the topic.
Laurens Liesenborghs: So we, we were completely surprised of course, euh, we expected, we, we knew about the epidemic potential, euh, about this disease, but then, going really to the far end of the world to look for Mpox cases and all of a sudden you come back and there are Mpox cases at your doorstep. So that was quite euh, quite strange indeed, and unexpected.
Narrator: You could start to wonder what drives such a disease. What is the reason a virus like Mpox suddenly appears in a population? It might start with someone eating a sick animal but why is it transmitted from human to human?
Laurens Liesenborghs: What are the important mechanisms? Is it through the respiratory road? Is it euh, because people get into close physical contact? Is it through euh, contaminating objects like a plate, or a spoon that is shared or clothes that are shared? To really understand these mechanisms behind euh, the transmission. And, because of course, that will enable us to stop these epidemics.
Narrator: So if we don’t map out our answers clearly in advance, before there is a crisis, we will steer blind during the outbreak itself. We would have no idea what measures to take. So it is of the utmost importance to research smaller outbreaks to collect data about the disease as soon as possible. But as the Ebola outbreak of 2014 made abundantly clear, researching an outbreak isn’t easy.
Dr. Mandjeku: Before we enter the Ebola ward, we pray.
Narrator: This is what Doctor Mandjeku tells us before we enter the ward.
Dr. Mandjeku: We put on scrabs, boots, a pair of gloves, foot covers, a full body Tyvek suit, a second pair of gloves, a respirator mask, a second hood, goggles, a third pair of gloves and a heavy yellow apron, not a spec of skin will be exposed to the air. After just 5 minutes you are saturated in sweat.
Narrator: Walking into an Ebola ward is a strange experience.
Johan van Griensven: The first time it’s a bit horrifying.
Narrator: This is Johan van Griensven, from ITM. Together with Alex Delamou, who studied at ITM and was doing his PhD in 2014, he was researching Ebola.
Johan van Griensven: You see people very sick dying, bleeding, euh, confused, you, to some extent have to keep distance. You feel a bit helpless, because also what you can do medically in such a facility. It has changed now, but at that point in time, it was basically about isolating them, euh, and giving some fluids and there was not a lot beyond that that you could do. Situations happened where Ebola patients would start running around and trying to escape and, and, yeah…
Narrator: Quite a challenge. And you are overwhelmed. On the other hand…
Johan van Griensven: You have to still think very clear, it’s very hot, you are sweating, you are dehydrating but you have to stick to the precautions. You might see someone vomiting, you might wanting to help, but then if the person is confused pulls your mask, you might actually get exposed yourself, so it’s finding a balance between keeping your head cool, but still yeah, they are still patients.
Narrator: And the only thing those patients see from Johan and Alex is them walking around in some kind of space suit. You have to admire the health care workers who do this day in and day out.
Johan van Griensven: I think they are, it’s where most of my respect goes.
Narrator: It’s a very high-risk job and if they get sick, they will just end up with the other patients.
Johan van Griensven: They are remarkable people.
Narrator: No matter how complex the situation in such an Ebola ward is, the reason why Johan and Alex are there is simple. They want to better understand the disease and this is by no means self-evident. An outbreak is always unexpected.
Laurens Liesenborghs: Of course this means that you need to drop everything and, and go there often without funding.
Narrator: Laurens again. This is one of the reasons why the Institute of Tropical Medicine has set up an outbreak research team. A multidisciplinary group with an anthropologist, a virologist, a clinical scientist, a lab expert, an epidemiologist and so on. They tried to be ready to study a disease as soon as there is a new outbreak. Alex Delamou dropped everything.
Alex Delamou: The Ebola outbreak started in my country, Guinea, I decided to go back and help with euh, Ebola control.
Narrator: Johan and the rest of his team wanted to go to Guinea as well as test a new treatment with plasma from blood of Ebola survivors. But they were not sure and had to decide which action to take.
Johan van Griensven: A dramatic outbreak can lead to political unrest. There was already violence, so unpredictable, even risk for the people involved. Are we up to it? Will we take the risk? Is everyone willing to take it?
Narrator: Eventually, they thought about it for three precious days.
Johan van Griensven: We called each other every day, over the weekend and on Monday we decided, we will proceed.
Narrator: This time, Johan and Alex arrived in time but the team had to be quick. Ebola pops up, kills a lot of people and it is very plausible that the virus will retreat back into the forest before they can get their studies up and running. Or before they can get to the remote places where the disease rages. It could be years before Ebola returns to humans, attacks and retreats before it can be studied. And there is another reason the team had to be quick. When an outbreak starts…
Laurens Liesenborghs: Well, then everyone yeah, wants to stop the outbreak as, as soon as possible.
Narrator: But that makes research challenging. Because once the outbreak is over, there will no longer be people to participate in their studies. Everyone is either cured or deceased.
Laurens Liesenborghs: But okay, that’s, that’s part of the game and, and that’s one of the things that makes outbreak research so, so difficult.
Narrator: The outbreak research team does not go out there to actually help people or stop a specific outbreak. They are there to research it. To understand how the disease works.
Laurens Liesenborghs : This is a bit frown upon upon by humanitarian organizations, because the response, that’s what is important and an outbreak you need to control it, euh, you need to take care of patients and that’s all 100% true, but the big thing that’s also very important to also do research.
Narrator: Research is crucial, to learn lessons from outbreaks and in the future support the humanitarian organizations and response teams.
Jean-Jacques Muyembe: An outbreak is always something that is accompanied by euh, panic and also remorse. And most, most of the time we are not prepared for that. It is like a surprise.
Narrator: This is Jean-Jacques Muyembe, the microbiologist and professor who we met in our first episode.
Jean-Jacques Muyembe: You can take euh, the good measures,or the, the bad measures. Euh, most of the time, it’s the bad measures we will take at the beginning. Yes. So, an outbreak, euh, it is like a, in a class, we are, we are learning. We are learning during the outbreak. To find medicine, to find vaccines, and also to change the behaviour.
Narrator: Johan and Alex knew they had to be quick. Reports of the Ebola outbreak were all over the news.
Reporter 1: There is a fear that the virus will spread rapidly.
Reporter 2: This is the deadliest outbreak of Ebola on record.
Reporter 3: Any communicable or infectious disease can go anywhere in the world within 24 hours.
Narrator: Johan didn’t know this at the time, but it would eventually become the largest Ebola outbreak ever recorded and would cost the lives of more than 11,000 people.
Alex Delamou: I must say that it was really a shock.
Narrator: Alex again.
Alex Delamou: We quickly realized that the country itself would not be able to respond, euh, because of the rapidity euh of this spread of the outbreak, euh, it started in one town and then, euh, in four, in four, five months it was already in the capital city. Euh, which is located about 1.000 kilometres from the place where the first cases started.
Narrator: And once Johan and Alex’s team had set up, nothing went as planned. They might have mapped out a strategy, but every day they realized their approach was not working as intended. Or that there was resistance. Problems stacked onto more problems.
Johan van Griensven: So it’s frightening and, and also yeah, stressful, definitely stressful. There is also very, a lot of pressure also, expectations locally, also politicians or ministries of health that support you. Euh, they, they want to help the community, they also want to show to the community things are being done. So you have to deliver.
Alex Delamou: But also you are doing it within an epidemic period. So you have to protect yourself and also you have to protect euh, your family and your, your close relatives you know. So it’s like euh, very, very risky area. So, you have to be euh, 100% euh, focus because small mistakes can break anything.
Narrator: Or as Laurens puts it when he was in the DRC…
Laurens Liesenborghs: If something goes wrong, it can go wrong quickly and, and very badly.
Narrator: Evacuation options are limited.
Laurens Liesenborghs: One night and there was a, a territorial dispute, euh, 20 kilometres from, from the village where we were remaining, so a territorial dispute between two villages.
Narrator: Rumors suddenly started to spread. An attack on the village was imminent. And what was more, in a recent unrest, one soldier was killed and one went missing. The weapons of those soldiers, two AK47s, were still in circulation.
Laurens Liesenborghs: So there were guns in, in the village and so, then all of a sudden things can get tense quite often.
Narrator: Then, you had to work as a team.
Laurens Liesenborghs: Being able to rely on each other is, is crucially important and so, so then I was very happy to be there with, with my Congolese friends, euh, which did an amazing job also to, to negotiate.
Narrator: The team got away safely. But it does indicate that outbreak research is not necessarily safe. Not for ITM researchers, nor for their local partners. Or as Johan summarises this Ebola project:
Johan van Griensven: I think, yeah, the most stressful moment, period of my life, I wouldn’t recommend it to my enemy. You live every second euh, you live for this project.
Narrator: But as a researcher, Alex says:
Alex Delamou: You have to take a responsibility to do your part of the job.
Narrator: An outbreak research team standing by to jump into action when there is an outbreak is crucial to prepare us for future cases. Because beyond the search for treatments and drivers of the disease, everyone wants to know where the virus comes from and where it could retreat to once it has run its course. In other words, where does the disease hide when it is not infecting humans?
Narrator: David is ten years old. He’s playing football with his friends. They hear the car before they see it. David forgets about the ball, drops everything and runs to the vehicle, it’s a 4 by 4. Some white people get out. Some Congolese as well. He doesn’t know they’re Mpox researchers, but he has heard some people will arrive in the village today. What are they up to? He keeps on watching them from a safe distance. The Congolese people speak the local language and talk to George, the father of one of his friends. After a while, George points them in the direction of the house of the chief. The visitors enter the building. David keeps on looking. But everything is quiet and nothing seems to be happening. David goes back to his football.
Laurens Liesenborghs: Generally, the reception is quite well, but of course, you don’t just arrive there…
Narrator: A visit to a village involves a lot of homework. Laurens and the team of Mpox researchers could upset the whole political and social balance in the village and that is the last thing they want to do.
Laurens Liesenborghs: Always work together and through local organisations.
Narrator: But also with governments or the chief of the village.
Laurens Liesenborghs: To make sure that, that you are welcome and to make sure and to inform people what the intention is and, and to also listen to what they expect.
Narrator: This is crucially important.
Laurens Liesenborghs: And that takes a lot of time.
Narrator: Laurens stays in the village for three weeks. He then returns to Belgium. His Congolese colleagues will stay longer. During those weeks, they will map out how Mpox is transmitted and they will be looking for the animal reservoir of the disease. A virus can only survive if it has a place where it can live happily and undisturbed. It must be able to reside in an animal that doesn’t die from the virus itself. Because when the animal dies, the virus also dies. Mpox for example indiscriminately infects monkeys, rats, mise, squirrels and rabbits. There is a good chance that the virus will withdraw into one of these animals when it is not infecting humans. But it could just as well be an animal that is not yet on our watchlist. Once Laurens and his colleagues know which animal is the reservoir for the virus, they can give advice on how to avoid contact with the animal and greatly diminish the chance for a new outbreak. Sounds deceptively simple but the search for the reservoir is not obvious.
Laurens Liesenborghs: It is like looking for a needle in a haystack. That is actually mainly by off the record research that you cannot do in a questionnaire, by really talking to people and knowing their hunting habits, that you understand yeah which species might be the culprit and so for example we have a lot of indications that it’s mainly squirrels that transmit the disease. But these hunting habits and these balances are, are quite complex.
Narrator: Once they have an idea of which animal is a contender, the team has to locate the suspect in that endless jungle, catch it and take blood samples.
Kevin Ariën: There is a lot of guidelines and regulations on this, you can not just go out and, and catch euhm, hundreds of bats and kill them off.
Narrator: This is Kevin Ariën, virologist at ITM, discussing the challenges of looking for the reservoir of Ebola.
Kevin Ariën: This has to be done in the least invasive possible way, euhm, with rodents, it’s a bit different but nevertheless, I mean, there is never the intention to just kill and slaughter these animals, it’s, it’s catching them, euh, taking a small blood drop and then releasing them again.
Narrator: The field researchers then send the samples to the lab where the virologist tests the blood to look for the virus. If the animal has the virus without getting sick from it: reservoir found. Or at least one of the possible reservoirs. Now researchers can write extra guidelines on how the disease is transmitted, and how to avoid contact with the animal. Sounds great, but it remains a big haystack and a small needle. Professor Jean-Jacques Muyembe is still looking for the reservoir of Ebola, almost 50 years after he first came across the disease.
Jean-Jacques Muyembe: We are studying also the reservoir of the virus of Ebola, but it is very difficult for the moment to have the proof, the scientific evidences, euh, to isolate the virus from the reservoir. But until now, we process more than euh, 10.000 samples at my institute, but we didn’t euh, isolate the, the virus.
Narrator: The challenge is that most of the time, samples aren’t collected during the outbreak itself.
Jean-Jacques Muyembe: So it is very difficult to isolate the virus, but we know that the first outbreak of Ebola Sudan, the patients were infected in a cotton factory. And euh, the roof of the factory was plenty with bats.
Narrator: And we also know that the reservoir of the Marburg virus, the cousin of Ebola, is a bat.
Jean-Jacques Muyembe: But until now, we have no confirmation for Ebola.
Narrator: We keep looking. Looking for some kind of pattern that can help us.
Narrator: A woman sits under a large and beautiful mango tree.
Kathy Kreppel: Usually on a little chair, under a big tree, hopefully it’s not gonna rain too much.
Narrator: The woman under the tree is Kathy Kreppel and she’s waiting. Kathy is an epidemiologist at ITM. She is here to talk, to talk to people and to find patterns. Kathy loves patterns. She recently found a special pattern that links the rainy season to the plague in Madagascar and it has something to do with traps and rodents.
Kathy Kreppel: I already found out that they have certain times when the kids are preparing for the rodent season and making all sorts of elaborate traps, it’s a bit like a competition, who has the best trap.
Narrator: And when rodent season starts, all the children enthusiastically start catching rodents and then they get sick.
Kathy Kreppel: Knowing that they do that at certain times of the year, to me indicates that there must be a seasonality. And seasonality is always linked to climate.
Narrator: If Kathy can find the right link between something as large as the seasons and something as small as a rat catching competition in Madagascar, she will finally understand which factors play a role in spreading the disease. Kathy knows she can’t ask people in the village why they start catching rodents at this time of year.
Kathy Kreppel: For them it’s very, it’s totally clear. They are like ‘well, this is when the rodents are out.’
Narrator: But why are the rodents out? She has to find the bigger link herself. Eventually Kathy realized it had to do with the end of the rainy season.
Kathy Kreppel: It’s when the rain stopped, so you have a lot of rodents because they have just had a lot of food, a lot of grass, and a lot of insects and so to eat. But now the rain stopped so you can start lighting fires and you can strategically set the fire, so it pushes the rodents that run away from the fire into your specially prepared traps. But of course depending on the climate, the rain will take longer or will not come at all or the dry season is very sudden.
Narrator: And all of that has an effect on the start of the trap making competition and on the timing of the spread of the disease. It also tells you who is at risk of contracting it.
Kathy Kreppel: It is not the grannies that are sitting at home, it’s the kids that are in touch with the rodents and catch them and kill them and get scratched and bitten.
Narrator: How do you beat an infectious disease? A good question. You can start with medicines, vaccines or lockdowns, you can take it apart in a lab to understand how it works or look for a treatment. Kathy chooses a different route. She tackles the disease with questionnaires, conversations and big data that link the big picture to the minuscule details. And that is why we now see her sitting on a small chair under a large mango tree, waiting for the village elders to tell her stories about how they live.
Kathy Kreppel: I love being in the communities and just having these aha-moments.
Narrator: By talking to the people, she can uncover little details that she would never ever think of on her own and that she can only see when she is in the village. She can then link these little stories to huge datasets, like linking the rats to the rainy season. But she also discovered that the outbreak of the plague in Madagascar is often linked to the El Niño Southern Oscillation, a climate phenomenon in the Pacific Ocean. Wait, stop. A natural phenomenon somewhere in South America has an effect on the outbreak of plague in Madagascar, an island that last time we checked, was off the coast of Mozambique? Yes, correct. In Madagascar.
Kathy Kreppel: There is a tradition to remember the dead every seven years and they actually take the shrouded, so they, they, they wrap their dead in shrouds, lay them to rest and after seven years, they take them back out. They are not in deep graves, they are more in like, a bit like a crypt, euhm, but above ground. So they take the shrouded euhm, dead out and they spend days just singing about their achievements and their lives to remember the dead. Euhm, it’s a really big traditional, very important festival.
Narrator: Unfortunately there’s evidence that while they do so, they also become infected with the plague, which comes from fleas from the rats that live in the crypts. And since the El Niño Southern Oscillation affects the weather and the weather affects how many fleas there will be on the rats, El Niño has a clear effect on the likelihood of a plague outbreak in Madagascar.
Kathy Kreppel: Things are interconnected, there is no black and white, there are many factors affecting things, especially diseases, it’s a complex system.
Narrator: During the outbreak itself, you can no longer find Kathy in the field or on a chair under a tree. During an outbreak she no longer concerns herself with individual cases. At that moment she remains at ITM in Belgium and mainly wants to be fed with data. Lots and lots of data.
Kathy Kreppel: So we would like to know what’s the climate data, what was the climate data in the last year. How many cases are there, what are the conditions right now? Was there anything special? It’s not about dropping everything, going there and seeing a patient. Because actually, the exact symptoms that a patient has is for me not very meaningful. That doesn’t, doesn’t play a role. What plays a role for me is how do the families react, how does the country react, euhm, what is the normal behaviour, the day-to-day behaviour of a person that got infected.
Narrator: In this way Kathy tries to get a clear picture of how the pathogen reacts in people and how people react to the disease. She says to other researchers and health professionals: give me the pieces of the puzzle. She then tries to put together everything we know and tell us what that picture looks like, for instance the picture of avian flu. At that moment, the outbreak itself has already started.
Kathy Kreppel: You can’t stop it from causing the first cases, it’s already happened. But you can prevent it spreading. With avian influenza you need to know where the birds are. You need to know where the poultry farms are. You need to know what the regulations in the country are. And so on.
Narrator: And so she tries to answer the question…
Kathy Kreppel: Who is gonna be next for example. What is gonna happen next?
Narrator: Whether you are Laurens stuck in the mud, Kevin peering through his microscope, Johan trying to set up a project or Kathy trying to solve the puzzle, it is very difficult to collect answers and puzzle pieces during the chaos of an outbreak.
Laurens Liesenborghs: It is crucially important to prepare well in what we call peace time, and this is also for research.
Narrator: Much better to collect your answers in advance so that you know how a disease will behave when it breaks out. Nowadays we have the solutions to cure a lot of infectious diseases. We often know how the disease is transmitted and how we should stop the outbreak. We sometimes even have vaccines. But there is one giant challenge. We can get all the biomedical proof we want, but if people don’t want to take a leap of faith, it’s useless. We need to know how people think and react in times of uncertainty. We may already find it difficult just to understand our partners or our best friends at times, so how can we expect to ever understand what drives people from other cultures or people with opposing opinions. That is what we will figure out in the next episode.
Charlotte Gryseels: The only thing standing in the way of malaria elimination is really the human factor.
Kathy Kreppel: There are many, many things that we do every day where we know that is not healthy, but we do it anyway.
Narrator: Thanks for listening. Join us next time and find out the importance of the human factor in combating infectious diseases. For more information on the Institute of Tropical Medicine in Antwerp, please go to ITG.be/podcast.
A leap of faith
Transmission #3
Why do or don't we take advice from healthcare professionals and authorities? What have previous outbreaks taught us about this "human factor"? Follow our researchers as they pitch their tent under snake-filled trees and talk to people in remote villages to find the answers.
A leap of faith
Transmission #3
Charlotte Gryseels: Where do you start? You start by talking to people, and that is also where you end, basically.
Narrator: This is Transmission, the podcast of the Institute of Tropical Medicine in Antwerp. In this podcast, we will uncover the mysteries of diseases that impact us all and delve into the cutting edge science of keeping people healthy. We invite you to look over the shoulders of the experts who make it their life's mission to improve global health. In our second episode we followed researchers in the field as they tried to unravel the mystery surrounding the transmission of infectious diseases. In this third episode of Transmission, we will discover that understanding humans is even more important than understanding the disease itself. Transmission, your front row seat to the world of health, science and beyond.
Narrator: A village on the island of Madagascar, Kathy Kreppel, epidemiologist from ITM, is setting up her tent with the help of children, a lot of children. She has found the perfect spot under a beautiful large mango tree. Thanks to this setting, her tent is in the shade and a little sheltered. After a while Kathy and the children have gathered quite an audience.
Kathy Kreppel: I had this whole audience, the kids putting up my tent, very happy, banging things everywhere. They had no idea how to put it up, they just tried their best.
Narrator: But there is something going on that she can’t put her finger on. At the edge of all this activity, the village elders are watching.
Kathy Kreppel: Just standing there, shaking their heads.
Narrator: They watch with the look of….
Kathy Kreppel: When is she gonna notice…
Narrator: But Kathy has no idea what to notice.
Kathy Kreppel: I was new, couldn’t speak proper Swahili.
Narrator: The tent has been set up. The grandmothers call the children back to watch the tent from a distance. The sun starts to set and then…
Kathy Kreppel: Snakes started falling out of the tree, onto the tent.
Narrator: Apparently, there are snakes that crawl up a tree during the day looking for small birds. When it gets cooler, they let themselves fall down on the ground.
Kathy Kreppel: They just slither down there and then just fall the last bit.
Narrator: The people of the village assumed that the educated woman who appeared in their village knew what she was doing. If she wanted to set up her tent under a tree filled with snakes, she could do that. Kathy learned two wise lessons that day. First, never pitch your tent underneath a tree, but above all…
Kathy Kreppel: The community usually is very helpful if you care to listen.
Narrator: You might sometimes forget when you are analyzing virus sequences in a lab or when you are trying to track down an obscure animal in the forest, that it’s the people who matter most when we talk about pandemics. And it is crucial to listen to them. Or as Charlotte, medical anthropologist at ITM, says…
Charlotte Gryseels: Where do you start? You start by talking to people. And that is also where you end basically.
Narrator: This is how you find out how everything is connected and how for example a small animal in the forest is linked to a worldwide outbreak. How small details in our behavior can affect everything. It’s people that give you clues on how a disease will behave.
Grace: Tonight, we eat a hearty meal with foo foo. Preparing the stew, is quite simple. It starts with the rodent. You peel off the skin and then, very important, you take out the insides. You don’t eat that. Here boy.
Narrator: Wait a second… This sound.
Grace: ‘Here boy’.
Narrator: That is what we were looking for. That is the sound that prompted Kathy Kreppel and Justin Mazumu to come to this village in the Democratic Republic of The Congo in search of the source of a major Mpox outbreak in the region. Justin and Kathy know that there is an outbreak of the virus.
Kathy Kreppel: We don’t know really when this transmission happens.
Narrator: What is the link between the rodents that carry the virus and the people who get sick? How does the disease spread? That is what Justin and Kathy wanted to find out. They had been in the village for several days before they noticed it.
Grace: Here boy.
Narrator: While cooking, Grace throws the guts of the animal she is preparing on the ground for the dogs to eat. Doesn’t matter if it’s chicken, rabbit or rat. Guts out and to the dog. Sometimes the children also play with the insides and cook pretend meals for the adults. It seems like an innocent act but Kathy and Justin knew they had discovered something. A key to the transmission of the virus. A sick animal caught in the forest or bought at the market and the guts go to the dogs or the children who play with them. Once you see it, it’s so clear. And most importantly, it’s something you can work with. Imagine you discover that not owning chickens is the best way to stop a virus. Then you are in trouble. No one will listen to you because chickens are an essential part of life. But asking someone not to give the guts of a rodent to the dogs, that is quite feasible.
Kathy Kreppel: It’s just an aha-moment, you know, they are like, oh, we didn’t know. So from now on, we will not feed that to our dogs. As easy as that.
Narrator: The challenge is that you have to get to the place where you can point your finger at that small detail. The moment you can say, that is the link we should look at more closely. Here is Charlotte again, medical anthropologist.
Charlotte Gryseels: So when you start every day you, you, you get certain insights or certain access to one piece of the puzzle and it leads you to the next clue.
Narrator: The most valuable insights come from the people who live where the outbreak is happening.
Charlotte Gryseels: They have such different practices surrounding what we consider the most normal things you know, euhm, they approach life so differently and I learned so much from them.
Narrator: Humans are the most challenging factor in our hunt for clues.
Charlotte Gryseels: We often joke actually in our unit that the only thing standing in the way of malaria elimination is really the human factor.
Narrator: Because people can give you clues on how the disease will behave, but they can also be stubborn and act against advice or better judgment. Why would they do that?
Narrator: We all know the advice on how to live longer and healthier. Eat well, sleep well, exercise. To support us in our quest for a healthy life, researchers developed the happiness triangle, the food triangle, the food circle and who knows what other shapes. So we know that it is important to exercise enough and eat less sugar. And yet, we don’t adhere to that advice. The truth is that we as humans do a lot of things throughout the day that we know aren’t healthy. You shouldn’t be ashamed of that, everybody does it. But the question is, why? Why do we ignore meaningful and good advice and do things we know are not smart? All over the world, we act against our better judgment. Just as it was impossible to stop Belgians from hoarding toilet paper during the Covid pandemic, it is also impossible to ask a large part of the world to stop buying food at wet markets where live animals are slaughtered and sold. It is their way of life. It won’t work if you try to change those key elements. Back to your drawing board, researcher. Find another solution. And so we get the golden nugget of this episode. The ultimate treasure that often explains why people follow advice or not. The thing every researcher is looking for: trust.
Kathy Kreppel: Imagine your wife gets really sick. And she is in the living room on the chair and you call the ambulance.
Narrator: You wait, and then, paramedics suddenly bang on the door and enter the room in white overalls. They tell you to not leave the house for the next week.
Kathy Kreppel: Nobody really tells you what’s going on, everybody is in emergency mode.
Narrator: People are busy discussing the situation next to your partner’s bed.
Kathy Kreppel: Would you just let that happen?
Narrator: That will very much depend on whether you trust those people in their white overalls or not.
Kathy Kreppel: We come from a society where we trust the government and the systems, but imagine you come from a country where you cannot trust the system and the governments as much as we can.
Narrator: Research shows time and time again that problems with medical interventions have little to do with a lack of knowledge about the disease. They have everything to do with the lack of trust. In times of crises providing more information is of little use if you want to convince people. You need to build more trust to get people on your side. Otherwise, if there is no trust and people just storm into your house…
Kathy Kreppel: You would get quite angry. You would try to stop them.
Narrator: Charlotte Gryseels and Soka Suon investigate why ethnic minorities in Cambodia resist using mosquito repellent in the fight against malaria. Soka graduated from ITM in 2021 with a master in public health and is now senior board program coordinator at the national center for HIV aids in Cambodia. But when he was doing research together with Charlotte, his main interest was malaria. Theoretically, mosquito repellent makes a lot of sense in the fight against malaria. Because there are no mosquitos during the day and you sleep under a mosquito net at night, the hours of nightfall are the moment when they will bite you most. By collectively applying mosquito repellent at nightfall, there will be much less transmission and much less chance to get sick. Can’t be simpler. Any child can understand. And yet, Charlotte and Soka see that people do not use their mosquito repellent. Why is that?
Charlotte Gryseels: I think minorities, yeah, they have actually a very different lifestyle from the majority Khmer in Cambodia.
Narrator: The people live in the forest and are completely self-sufficient. They are often not registered as residents of the country and do not speak the language.
Charlotte Gryseels: So they have this really difficult relationship with the state, with the Cambodian government.
Narrator: They value their independence very much.
Charlotte Gryseels: So when Cambodian and foreign euh, researchers euh, scientists come in and give them repellents to use every day between that and that hour…
Narrator: Then they don’t trust those researchers’ motives. Can you blame them? They associate the scientists with the authorities and trust is minimal. And so, they don’t put in the effort in applying mosquito repellent. The first step to do research in this setting is therefore building trust. And you do that by living in the village. Working together with the residents, drinking and eating together or attending wedding parties.
Soka Suon: They feel that we are part of the villagers there.
Narrator: This is Soka Suon, alumnus of ITM and Charlotte’s colleague.
Soka Suon: And we go back and go back and go back, so they feel more confident with us, so, we stay more than six months.
Narrator: All of this to have that meaningful conversation that goes beyond mistrust or general statements. And that is no different in Belgium. Establishing a constructive dialogue with the people who had doubts about Covid 19 vaccination.
Charlotte Gryseels: … proved to be a task that nobody was, was up for.
Narrator: What was striking was that the conversations around the vaccination topic actually broke down trust and widened the gap between groups. While researchers had shown time and time again that scientific evidence and fact checks are not the right way to build trust, it often seems to be the only approach during a pandemic.
Charlotte Gryseels: And what I found particularly problematic and difficult to deal with as a researcher, but also as a person, euhm, is that experts and politicians also started framing euh, vaccine refusing individuals as dangerous, as misinformed, as unintelligent, as euhm, selfish, overemotional.
Narrator: Conspiracy theorists, scientifically illiterate, there were a lot of negative words for people who doubted. And this naturally widened the trust gap which created even more resistance.
Charlotte Gryseels: Seeing this unfold, in real life, in my own society was quite difficult and it was obvious for me that that’s definitely not the way to approach people during an outbreak.
Narrator: There are no easy answers. If scientists are very transparent about their info and data…
Charlotte Gryseels: Then people actually see that science doesn’t produce hard truth.
Narrator: On the other hand, if scientists aren’t transparent, people won’t trust them either because they withhold information.
Charlotte Gryseels: I don’t have a way to resolve that paradox, I don’t know what’s better.
Narrator: It all has to do with taking a leap of faith. When there is a crisis, the less people on different sides trust each other, the wider the gap and the bigger the leap people have to take to listen to advice from the other side. The challenge is that this gap in trust only widens during a crisis, right when policy makers and scientists ask the population to trust them to implement the proper measures.
Charlotte Gryseels: You are not relying on an anthropologist to start building trust during the crisis cause that’s not gonna work. It takes a lot of time.
Narrator: We need to get used to the fact that science is always in motion and we need to invest in building trust in periods of calm. Building it up, step by step, so we can lean on it in times of crises. Jean-Jacques Muyembe, who was one of the scientists who first discovered Ebola and whom we met in previous episodes, elaborates.
Jean-Jacques Muyembe: Changing the way of view of other people is the most difficult aspect of an outbreak. An outbreak of Ebola starts most of the time by the amplification of the disease in a hospital. The second place is, is the funeral in the community. But in the community, to change the, the behavior of the population is, is very difficult. Yeah, because they want to go and kiss the cadaver, wash the dead body. They have no gloves and so on.
Narrator: To prevent people from getting sick during the first Ebola outbreaks, families weren’t allowed to participate in the funeral. It was conducted by the Ebola team.
Jean-Jacques Muyembe: This was a very bad decision in the community and later what we have proposed is to have the participation of the community of the, of the affected family, so they were given protective equipment, gloves, so, they can take the coffin with the member and bring this to the cemetery. And, and sometimes also they, they will refuse the countermeasures proposed by the Ebola team. So if they not accept, this a very, very, very, very complicated situation.
Charlotte Gryseels: And there is just no quick fix for trust issues, there is no finish line, there is no, it always kind of a very volatile process, things can go wrong all the time.
Narrator: We are back in mosquito country. Charlotte is still looking for ways to gain trust with the residents of the remote village in Cambodia to try to find out why applying mosquito repellent is such a challenge. It isn’t easy. People don’t open up to her. Conversations are difficult. But lucky for her, this is all about to change. The downside: she will become sick, incredibly, miserably sick.
Charlotte Gryseels: At, at a certain point I got really, really inexplicably ill. And even my, my Cambodian colleagues were really quite worried about me and I mean, I had constant diarrhea. I was losing weight very fast, I, I had, I couldn’t eat anymore, I was very weak.
Narrator: A worrying situation, but then something happened that she hadn’t expected.
Charlotte Gryseels: I started learning a lot about how people perceive illness.
Narrator: In the first months after she arrived in the village, people didn’t feel comfortable talking to her about their religion, about wicked witches and spirits in the woods. But now Charlotte was sick, the situation changed. The medication she got didn’t work and she became weaker and weaker. People were concerned and started to talk to her. Maybe she was bewitched. By being ill, a world that previously remained invisible, came into focus.
Charlotte Gryseels: Suddenly when I was really ill myself and I was trying to find some medicines that worked I visited basically all the little shops and the pharmacies along the road and euhm, then they started opening up to me about the all the medicines they had, euhm, about all the unregulated often fake medicines euhm, illegal medicines probably that, that they thought might, might be able to help me.
Narrator: Previously, Charlotte had no idea.
Charlotte Gryseels: I didn’t even know that I had to ask questions about this.
Narrator: But thanks to her illness she became part of the village, part of the group. The entire medical system became clear to her.
Charlotte Gryseels: Seriously, in the entire province there was no doctor, I mean, there was maybe one health centre with a nurse that has some you know, malaria rapid tests, but there is not a lot they can do. So, if you are sick, you are really, kind of screwed. And then I started seeing how it completely made sense in a way to just blame it on a witch or you know, euh, cutting down the wrong tree in the forest. If you can’t do anything else about it anyway, then, then the system kind of works to, to make it more bearable.
Narrator: Her Cambodian colleagues were also concerned.
Charlotte Gryseels: Cause they also thought okay, you, obviously the medicines aren’t working, so, you have been bewitched you know.
Narrator: No one managed to make a correct diagnosis of what disease she had contracted. Was it a bacteria, a parasite, a virus, or something else? Both her Cambodian colleagues and the researchers at ITM in Belgium were in the dark. After a battery of treatments she was able to eat again. But it took her more than a year of severe diarrhea and fatigue before she eventually recovered.
Narrator: One of the Cambodian colleagues that helped her through this difficult time was Sambuni Uk. Someone who Charlotte thanks on the first page of her doctoral thesis and with good reason.
Charlotte Gryseels: I owe everything to her actually.
Narrator: During their research, ITM scientists are often totally dependent on their partners. These are usually researchers from a local partner organization who jump in and support the research project.
Charlotte Gryseels: Sambuni Uk, my euhm, colleague in Cambodia who was also a medical doctor and a social scientist, euhm, she worked at the National Malaria Centre, I just completely physically basically relied on her, you know.
Narrator: Sambuni took Charlotte in when she arrived in Cambodia as a starting researcher, and gave her food and a room in her house. She even made a ten-hour drive to get Charlotte to the hospital when she was so sick that she needed to be hospitalized.
Charlotte Gryseels: She had dinner with me every night in the field, so I wouldn’t be alone, so, euh, most importantly maybe: she showed me how to go to a toilet in a village where there are no toilets and there is no water, and there is no electricity, all those little things are, are very important actually. We as field researchers we also, we often try to portray ourselves as these kind of Indiana Jones type adventurers who have no problem euhm, getting by in these difficult conditions. But it’s actually it’s really, really hard living in a village without any electricity or water when it’s euhm, 45 degrees out, also at night, and I really wouldn’t be able to do it without the help from euhm, colleagues in the country really. Without their informal, social support, we really wouldn’t be able to do what we do.
Narrator: Again, it’s all about people. Without people, nothing would work and no outbreak would be stopped.
Narrator: We’ve put together the puzzle. We’ve sent team members all around the world to look for the reservoir of infectious diseases, had a virologist analyse the virus in the lab, looked for the drivers that multiply the disease and most important of all, we now have a better idea of the human factor. For important diseases like Ebola, Zika or Mpox, there are still a lot of important questions that remain unanswered. But let’s imagine for a moment that we have all the information we need, all the answers to every question we are looking for. Are we now ready for the next pandemic? Let’s find out in the final episode of this season.
Kathy Kreppel: All epidemiologists were going like, thank God, nothing happened. But they also went like, so why did nothing happen? Why did it not go anywhere?
Narrator: Thanks for listening. Join us next time when we will find out if humanity is ready for the next outbreak. For more information on the Institute of Tropical Medicine in Antwerp, please go to ITG.be/podcast.
The next one
Transmission #4
When it comes to the next pandemic, the question is not "if" but "when". In this compelling episode, you will meet the most likely candidates for the next outbreak, and find out how well-prepared we are to tackle it. We invite you to explore potential scenarios and reflect on our learnings.
The next one
Transmission #4
Laurens Liesenborghs: We never know what would be the next outbreak or, or even pandemic but there, there will be one, that’s for sure.
Narrator: This is Transmission. The podcast of the Institute of Tropical Medicine in Antwerp. In this podcast, we will uncover the mysteries of diseases that impact us all and delve into the cutting edge science of keeping people healthy. We invite you to look over the shoulders of the experts who make it their life's mission to improve global health. In the third episode we discussed the importance of the human factor in the transmission of infectious diseases. In this final episode of the first season of Transmission, we will answer the ultimate question: are we ready for the next one? Transmission, your front row seat to the world of health, science and beyond.
Kevin Ariën: We made a few steps I think with, with this Corona pandemic but are we ready to, to prevent a pandemic? No. And I, I think we will never be ready to, to fully prevent a pandemic from, from emerging.
SFX Reporter man: This is believed to be ground zero in the swine flu outbreak.
SFX Reporter woman: The world is now at the start of the 2009…
[SFX news flashes]
Narrator: In 2009, swine flu, formerly known as the Mexican flu, made its debut into the world. It was an adventurous flu virus that took the leap from pigs to humans and had the possibility to cause an epidemic. Pigs are infected by the same influenza viruses that can infect humans. Viruses such as H1N1 for example. Jumping from pigs to humans is not such an illogical move for a virus. Humans and pigs have quite a lot in common. We share a lot of DNA and we are even experimenting with organ transplants from pigs to people.
Kathy Kreppel: And everybody got really scared, everybody was interested in the topic, got a bit like oh, what is gonna happen now? What are we gonna do?
Narrator: Kathy Kreppel, epidemiologist at ITM. There was anxiety. Swine flu was something you discussed in the pub or at work. The World Health Organisation sent out warnings. Everyone was on edge and then…
Kathy Kreppel: And then nothing happened. And everybody forgot about it.
Narrator: And people were angry at the World Health Organisation.
Kathy Kreppel: They said there gonna be a big pandemic and lots of people will die and then nothing. And actually, I mean, all epidemiologists were going like, oh, thank god, nothing happened. But, they also went like so, why did nothing happen? Why did it not go anywhere? Euhm, why did it not kick off in a big way? What are the things that stopped it? Euhm, and how can we learn from these things to stop it next time?
Narrator: When a new disease hits, there is a sudden peak of interest in outbreaks and a surge in research funding. But both quickly fade when the disease starts to dwindle. Leaving us unprepared for the next one.
Narrator: In 2013 an asteroid exploded in the atmosphere above the town of Chelyabinsk in Russia. It did so with the force of 13 nuclear bombs. More than 7.000 buildings were damaged and 1.500 people were injured. And what is more. We didn’t see that asteroid coming. It was a complete surprise. If the cards had been stacked another way, the rock from space could have done a lot more damage. Stones from space sometimes skim past the earth without anyone noticing and sometimes can mean the violent end of the dinosaur era. Not very different from diseases. Swine flu was a near miss in 2009. But near misses happen more than you think. And the world of infectious diseases is full of surprises. Mpox surprised us and no one saw Zika or Covid coming. But in the past years, there were also outbreaks of Lassa virus, Bolivian hemorrhagic fever, Hendra virus, Nipah virus, Marburg virus, West Nile virus and many, many more. We sometimes feel that the threat of a pandemic is behind us now that we have Covid more or less under control.
Laurens Liesenborgs: Well think again and it can always come from unexpected places.
Narrator: This is Laurens Liesenborgs, infectious disease expert at ITM.
Laurens Liesenborghs: So we never know what would be the next outbreak or, or even pandemic, but there, there will be one. That is for sure.
Kathy Kreppel: We had a lot of cases that unfortunately don’t come into the media so much, where there was a very scary case, but the outbreak didn’t happen.
Narrator: … says epidemiologist Kathy Kreppel.
Kathy Kreppel: And we also need to understand that: why something does not kick off and happen.
Narrator: Because an outbreak that will hit the mark and spread to a worldwide pandemic is coming.
Kathy Kreppel: Left, right and center;. We have, we have the avian influenza outbreak.
Narrator: But also, Ebola, Chikungunya, Monkeypox and many others that are knocking on our door. The drum beats louder and louder.
Kevin Ariën: Most international organizations, WHO, euh, surveillance networks were focussing on avian flu and the pandemic was caused by coronavirus. If there is really a new pathogen we will not see it coming.
Narrator: Outbreak research is essential to prepare us for the next one. But from which side should we expect the next shot? Some viruses we better keep a closer eye on, like H5N1. More familiar to you and me as bird flu. We tend to minimize the flu, but in a perfectly normal year, about 250.000 people worldwide die from the human flu virus. It’s a very dangerous virus at best. At its worst moment, it’s devastating, like the Spanish flu of 1918 which killed about 50 million people in one year. The big risk with bird flu is that it would adapt to thrive in humans. And that is not so unrealistic. Flu is an RNA virus. These are viruses with a single strand of genetic information instead of the more common double stranded DNA. This allows RNA viruses to mutate faster than any other creature on earth. They have a trick to accomplish that feat. A flu virus consists of different pieces of RNA.
Kevin Ariën: And these viruses can exchange this genetic material. So if two flu virus particles infect a cell, they can exchange some of their genetic material, and basically form a, an entirely new genetic constitution.
Narrator: And the new virus is born in the blink of an eye. And that is exactly what virologists are so worried about when it comes to bird flu viruses. Up until now, the H5N1 bird flu virus is not fully adapted for transmission from human to human. It can be transmitted from birds to humans occasionally but doesn’t thrive in our bodies. So cases mainly remain isolated to people who are in close contact with birds. Lucky us, because between 30 and 50% of all people infected with bird flu die of the disease. That’s very different from the 2% with Covid or the regular flu where casualties remain well below 1%. So we are lucky for now. But imagine for a moment that at some point…
Kevin Ariën: One person is infected with human flu and euhm, and gets infected with this H5N1 virus.
Narrator: If that happens, the patient is infected with both the bird and the common flu virus. And then H5N1 can get a shortcut. If it has two strains of flu which exchange genetic information and transform into a new flu virus, you get a new potentially very deadly and easily transmittable virus.
Kevin Ariën: And that’s I think where, where most of us are, are a bit worried at this point, euhm, the more this virus is circulating, the less we have it under control…
Narrator: And that is why you should really coop up your chickens when they ask you to. So that your chickens don’t get bird flu from wild birds. So that consequently you don’t get bird flu from your chickens and so that in the end you can’t be a walking vessel where common flu and bird flu meet to start a new global pandemic.
Narrator: So the question isn’t, will there be another, the question is…
Kathy Kreppel: Can we handle it? That’s a very different question.
Narrator: It’s impossible to stop the perfect storm, says Kathy.
Kathy Kreppel: It’s more about reducing the risk, controlling, being prepared instead of fixing, stopping, preventing, we are more like: we do our best.
Narrator: Isabel Brosius, infectious disease expert at ITM and part of the outbreak research team, confirms.
Isabel Brosius: Because you can do all the research that you want, you can have all the treatments that you want, you can have everything in place, but if you can’t convey the message and if people can’t trust what you are saying, then you won’t be able to even do anything because to control an infectious disease outbreak, you really need the people, yeah, that are at risk to be on board with this.
Kathy Kreppel: The handling has a lot to do with behavior, with attitude, with politics, with money, with what we think we are entitled to.
Narrator: Let’s face it, we are not ready for the next pandemic. But after every outbreak we learn from our mistakes and from our victories. Way too slowly and in small steps, but we learn by living through different outbreaks, like the Mpox outbreak in Belgium.
Isabel Brosius: The first things we did when we picked up these signals, was to be ready in the sense of okay, what do we do if a patient would present?
Narrator: You need to work in parallel, in real time to make sure that the care for patients goes hand in hand with the gathering of information.
Isabel Brosius: You need to try to learn as quickly as possible what is going on, who is at risk, how is this spreading. At the same time, we had a lot of practical things just to organize. We didn’t know at that time, like, how deadly might this disease be, euh, exactly how transmissible would it be. So you need to take precautions. So you have to figure out like how do patients come in, can they sit together with others in the, in the waiting room or not, or, how will the samples go to the lab for instance, preparing things like frequently asked questions to put on the website to make sure that potential patients would be informed, that other stakeholder groups would be informed, there is so many things going on at the same time. Yeah, there is a lot of really practical stuff to be organised.
Narrator: We urgently need more outbreak research to prepare us for the next one. Research like Johan van Griensven and Alex Delamou were doing in Guinea to try to get a better understanding of Ebola.
Johan van Griensven: We had two chairs, very old chairs in front of the Blood Transfusion Service. We would sit there like in the Muppet Show.
Narrator: Like Waldorf and Statler, the two grumpy men from the Muppet Show, Johan van Griensven and Alex Delamou would sit in their chairs and think about their priorities.
Johan van Griensven: What is top priority if we don’t solve this today we go home.
Narrator: In their moments of rest in an otherwise very chaotic time.
Narrator: In December 2013, a two year old toddler from a small village in Guinea was infected with Ebola after coming in contact with a bat. It’s the start of the largest Ebola outbreak in history. In March, three months later, there were 49 confirmed cases and 29 deaths. By July, the disease had spread to three countries. It’s the first time that Ebola had broken out of its isolated rural origins and had begun to reach densely populated areas. Everyone held their breath, as no one at the time knew how the scales would tip. Never before had this disease had so many opportunities to transmit itself to other people. One month later, in August, the WHO labeled the situation a public health emergency of international concern - a label reserved only for events requiring an immediate international response. During the following months, Ebola spread to seven more countries from Mali to Italy and from Spain to the United States. Ebola had jumped continents. Belgium appointed former ITM researcher Erika Vlieghe as national Ebola coordinator. Everyone was in full outbreak mode. Yet two and a half years after the first case was found, the disease disappeared into the forest again, leaving more than 11,000 people dead. Nobody saw it coming as the next pandemic. Nobody expected it to be so big. But it was. And somewhere in the midst of all the chaos of Ebola outbreaks and transmissions, Alex and Johan are sitting on a chair in front of a blood transfusion centre in Guinea. They want to know if plasma from Ebola survivors can help in the fight against the virus. It’s a project that always seems to raise more challenges than they can solve. And sometimes it’s just best to sit in the chair for a while to get your prioritIes straight. One of the challenges Johan and Alex were facing was the production of plasma itself. That seemed a tough nut to crack.
Johan van Griensven: The national blood transfusion service had been receiving very little funding, so the basic facilities were very limited.
Narrator: But they needed a place to produce the plasma. Because without plasma, no plasma therapy. It seemed like one of those problems that would be impossible to solve. Until one day the head of the blood transfusion centre received a phone call. It was the minister on the phone.
Johan van Griensven: An airplane has arrived containing a big container.
Narrator: The container was fully equipped with everything on board to organise plasma collection according to the best standards.
Johan van Griensven: You are struggling how to organise it locally and suddenly there is a huge truck with top notch equipment arriving.
Narrator: One of the many problems solved. Time to start the day and tackle the other challenges.
Narrator: An outbreak like Ebola is always unexpected. If you want to do outbreak research or learn lessons for future outbreaks, you have to be quick. You have to drop everything, travel to the scene and do it faster than you have the time to arrange things properly.
Johan van Griensven: For the first three months, we took money with us in our underwear. There was no system to, to transfer the money.
Narrator: Sometimes Johan and Alex exchanged money in a bank and walked out with 10 kilos of banknotes in their pockets. Money they needed to run their everyday life in Guinea, but also money that needed to be counted and managed. It was a hassle. A funny story maybe? No, Johan says.
Johan van Griensven: It creates a lot of stress. At that point in time it’s not funny. Euh, afterwards it’s funny, but at that point in time, yeah, there are so many other things you have to do urgently.
Narrator: Researching outbreaks is a challenge for everyone.
Johan van Griensven: It felt a bit like you are dropped in a warzone without arms. But you have to survive. And that is what we did every day. It was very stressful, yeah. So I don’t think it’s healthy.
Narrator: Outbreak research might be heavy, but every outbreak sets us on a path to a better understanding of these events.
Johan Van Griensven: So if you look at it in a positive way, yes we learn each time. If you look at it in a negative way, we could have learned much more.
Laurens Liesenborghs: I think that there is, there is a lot of hope.
Narrator: Says Laurens Liesenborghs.
Laurens Liesenborghs: And especially from Africa where we see in our collaboration with African partners, I think a lot is changing.
Jean-Jacques Muyembe: Yeah this is also my main objective, it was to, to create euh, critical mass of researchers. So when I started I was only PhD and when I start my priority was to train a lab technician.
Narrator: This is Jean-Jacques Muyembe, who was one of the scientists who first discovered Ebola.
Jean-Jacques Muyembe: So I sent some of them here at ITM, other went to euhm, United Kingdom, in London and euh, and euh also in the Institute Pasteur in Africa. So by cooperation I have a lot of partners here in Europe, United States, in Japan and, and, and so on. So euh, I sent euh, some of them, and now they are back because at INRB we have also a very cool environment for research. So they decided to come back. And now we are more than 30.
Narrator: Recently, Muyembe and a team of researchers even discovered a treatment for Ebola. A long-standing dream of his.
Jean-Jacques Muyembe: If I remember well, when I was euh, PhD student at the university of Leuven, my will was to treat mice that were infected with a different virus, so when I returned to DRC, my dream was to, to see, can I put into practice my expertise, to treat human who are infected with virus. Ebola was for me this opportunity.
Narrator: For decades, Muyembe worked on this mysterious disease, until recently they discovered a cure.
Jean-Jacques Muyembe: When we euh, treated the first two patients, the result was extraordinary. Euh, two patients received only one injection and when they later, they asked to eat, so they were able to eat. And after that, we treated euh, eight other patients and it was successful. So it was fantastic. And after that it was euh, approved by FDA in the United States as a treatment of euh, Ebola. For adults and for children. So euhm, for me, this was my dream, euh, this dream is now reality. Ebola is a curable disease.
Narrator: There is an enormous build up of expertise in the countries with which ITM cooperates. Not only in Africa, but also in Asia and Latin America. It’s expertise coming from trained people who are in contact with outbreaks day in and day out. And that is hopeful.
Jean-Jacques Muyembe: So the time we have now is the time to prepare, to prepare, to train people in the world. Maybe we will not be very, very ready but it is, it is a good preparation.
Alex Delamou: The first time I came to Belgium, I was a young medical doctor. And then I got training from Belgium in masters level and also PHD-level.
Narrator: Says Alex Delamou, the alumni of ITM who worked with Johan van Griensven during the Ebola outbreak of 2014. Alex went back to Guinea and used that knowledge.
Alex Delamou: To improve things in my, my country now, I am euh, full professor in my university where I teach, where I share the knowledge that I learn in euh, in euh Belgium and in other settings.
Narrator: Alex is now senior researcher in Guinea and professor of public health at the Africa Centre of Excellence. He now combines a good understanding of outbreaks with a deep expertise in public health. Making sure our health systems can withstand the next pandemic and won’t break down. Doctor Muyembe again.
Jean-Jacques Muyembe: It is important to strengthen the capacity of African countries because I think the next pandemic will come from Africa. So if Africa is able to detect early, it will be the best of all the world. But if Africa is not strong, the disease will spread quickly in the world.
Narrator: We need strong health systems and a network of partners to be connected and to share results quickly.
Jean-Jacques Muyembe: This is very, very, very important. And also to have some kind of equity, like with Covid, we didn’t receive reagents because all the reagent was euh, sent to industrialized countries in Europe, in United States and so on. Even the vaccine, they always say first our people here in Europe and after that we’ll send to Africa. So this kind of equity is very, very important to have. Not only for vaccine but also for reagent, for diagnostic, and also euh, medicine and, and so on.
Narrator: So will there be a next pandemic? Yes. Do you need to lie awake at night worrying about an outbreak?
Laurens Liesenborgh: Don’t let the possible threat of a new pandemic euhm, define the way you live. Don’t do that. But just be aware that, that this Covid pandemic is not, is not a single event.
Narrator: Every day, people are working on managing outbreaks. From the social scientist in Cambodia to the researchers stuck in the mud in the Democratic Republic of the Congo and from the virologist in the lab to the epidemiologist behind her computer or the researcher in a tent underneath a tree filled with snakes. Monitoring systems are set up all around the world, it’s going to be an exciting time. Experts are keeping track. Let’s hope they are ready to sound the alarm when the time comes. In the next season of this podcast we will travel around the world again in search of new stories and surprising discoveries. The whole world is our playing field and we have to collaborate, because if we don’t work together to improve health systems across the globe…
Alex Delamou: Then euh, we should not be surprised that problems will come from where the health systems are weak.
Narrator: If diseases emerge in vulnerable health systems and then spread rapidly around the world, how do you ensure that the whole world is on board? How do you ensure that health systems and the people who work in them do not crack under the chaos? That is a story for next season.
Narrator: Thanks for listening. This was the final episode of this season of Transmission. Please join us next season. For more information on the Institute of Tropical Medicine in Antwerp, please go to ITG.be/podcast.
Intermission
Discover how diseases leap from animals to humans, where they lurk between outbreaks, the critical role of trust in fighting epidemics, and how disease surveillance safeguards against future pandemics. Intermission can be viewed separately, or as the background to Transmission.

Bites, sips & breaths
Intermission #01
With Luciana Lepore, infectious disease specialist in the Unit of Emerging Infectious Diseases
Bites, sips & breaths
Intermission #01
Transcript coming soon.

The reservoir
Intermission #02
With Eugene Bangwen, biomedical scientist in the Clinical Reference Laboratory
The reservoir
Intermission #02
Transcript coming soon.

It's a trust thing
Intermission #03
With Nandini Sarkar, health psychologist in the Unit of Equity and Health
It's a trust thing
Intermission #03
Transcript coming soon.

Preparing for the next pandemic
Intermission #04
With Philippe Selhorst, medical virologist in the Unit of Virology
Preparing for the next pandemic
Intermission #04
Transcript coming soon.
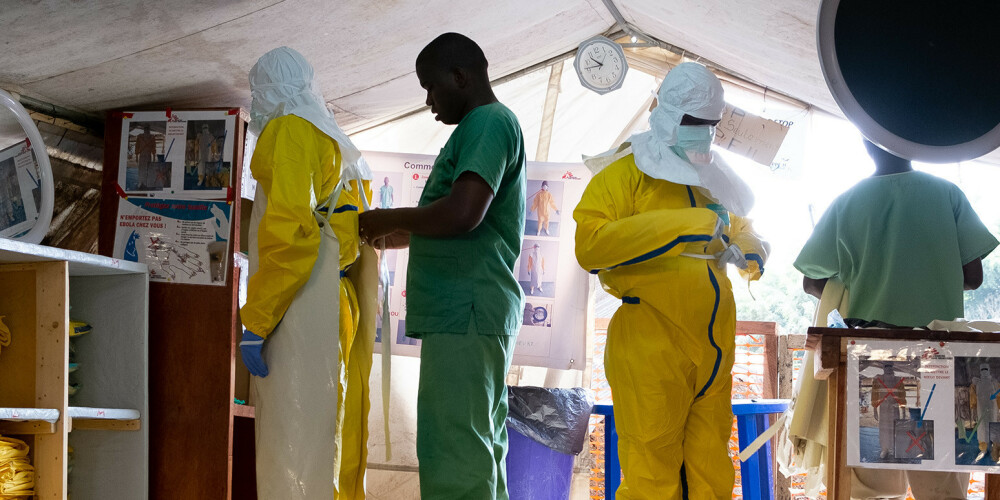
Emission
In today's interconnected world, emerging infectious diseases and outbreaks are a significant threat to global health. Our dedicated researchers work diligently in the field and in the lab to understand these pressing health threats and prepare for the next pandemic. Delve deeper into the science of outbreak research.