GeKoSkimm
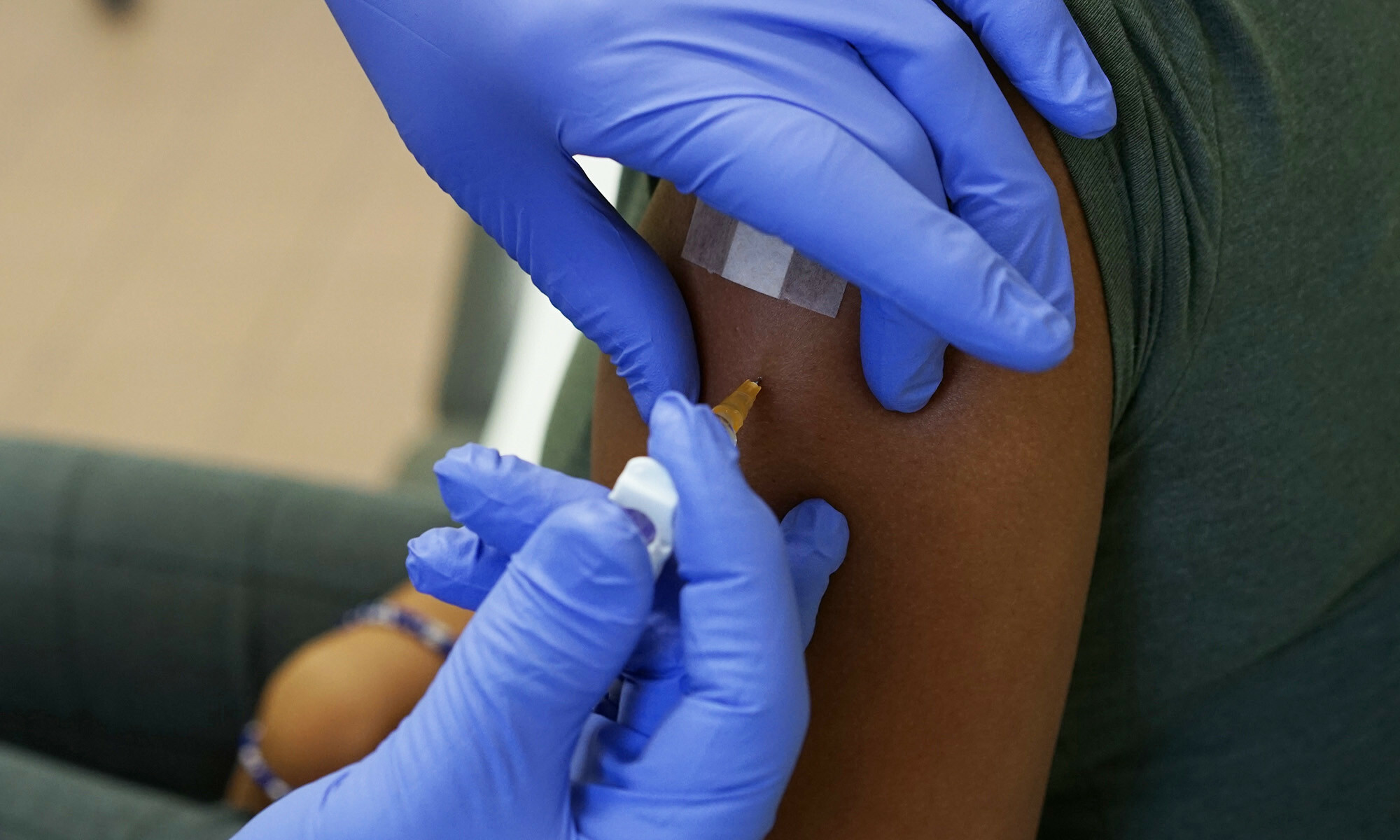
The Clinical Trial Site of ITM is seeking volunteers for a new clinical trial entitled GeKoSkimm. In this study, we will investigate the effect of yellow fever vaccination on the immune system of the skin.
For whom: Healthy volunteers between 18 and 50 years old who never received a yellow fever vaccination before
Start: Fall 2024
Duration: 4 months
Study visits: 4 visits to the ITM Clinical Trial Site
Intervention: Marketed yellow fever vaccine
Compensation: Between €350 and €550 for completing the whole study (depending on the number of optional biopsies)
Purpose of research
The yellow fever virus is mainly transferred through the bite of an infected mosquito. Symptoms manifest with a spectrum of severity, ranging from mild flu-like symptoms to severe hepatitis and hemorrhagic fever, often leading to death.
The skin – our largest organ – is the primary barrier after a bite accident. However, little to no research is done on the skin-specific immune response after vaccination, as conventionally we look at antibodies in the blood. This is, however, very important information for vaccine development against bite infections transmitted via mosquitos, but also flies, ticks, or even mammals.
Therefore, in this study, we will investigate how different routes of vaccine administration generate an immune response against yellow fever in both your blood and skin.
Who can participate?
We are looking for 222 healthy participants between 18 and 50 years old, who never received a vaccine against yellow fever before.
Conduct of the study
When you are invited to participate in the study, you will need to undergo a screening during the first visit, in which the study will be fully explained to you and in which the site personnel will determine if you meet all the criteria for participation. Subsequently, a blood sample will be taken and you will be randomly assigned to one of the three study groups:
Vaccination in the muscle
Vaccination into the fat layer under the skin
Vaccination into the skin
At each study visit, a blood sample will be taken. In addition, one month after your vaccination, to measure the immunity in the skin, a small sample of your skin (4mm in diameter) will be taken, close to the injection site. As you will be locally sedated, this is a painless and routinely performed procedure that the study doctor will explain to you during your first study visit. You will also receive materials to expedite the small wound healing and, as much as possible, avoid scar formation. You will have the choice to have a second biopsy taken the same day, and a third one during the last study visit 4 months after your vaccination. These two biopsies are optional.
To assess less invasive tools in future studies, we will also study a microsampling device. During this procedure, a 0.5mm microbiopsy will be taken (simply mimicking the bite of a mosquito) during the third study visit.
In order to keep track of any potential side effects of the vaccine, we will ask you to keep a diary for 28 days after your vaccination.
The vaccine that will be administered is approved, marketed and used worldwide on a daily basis.
Potential risks and inconveniences
Being involved in this study implicates that you will have a small amount of blood drawn at four timepoints. The total volume of blood drawn represents no risk to your health. Only the blood draw itself may cause some discomfort and possibly a bruise.
The vaccine may contain ingredients that trigger an allergic reaction. If you reacted poorly after vaccination before, participation is not recommended. The study doctor will discuss this with you during the first study visit. Except for the usual possible temporary side effects of vaccines, no additional risks or disadvantages are expected in this study.
The skin biopsies may cause some minor discomfort but are generally well tolerated. Any discomfort usually subsides quickly. The risks associated with these biopsies are minimal and can include minor bleeding, bruising, scarring or infection.
Reimbursement
To compensate for your time, any expenses you must incur to participate in the trial and possible other inconveniences, you will receive 50 euro per study visit and an additional 150 euro for the mandatory skin sample. If you agree to the optional biopsies, you will receive an additional 50 euro for the second sample and 150 euro for the third sample. Reimbursement will be done by means of bank transfer or gift vouchers.
If you commit to participate in the study, we strongly urge you to complete the whole study. However, know that you are free to end your participation at any time.
Team
Principal Investigator: Prof Dr Patrick Soentjens (MD, PhD)
Coordinating Investigator: Prof Dr Wim Adriaensen (PhD)
Questions?
Contact us at studies@itg.be.
Interested in participating?
Click on the link below to review the main criteria for participation. If you believe you are eligible, you can immediately register. Please note that this does not mean that you will be automatically selected to participate in the trial, as other factors depend on it. We will contact you anyway.
Share this trial on