Strengthening clinical trial review and oversight in Uganda


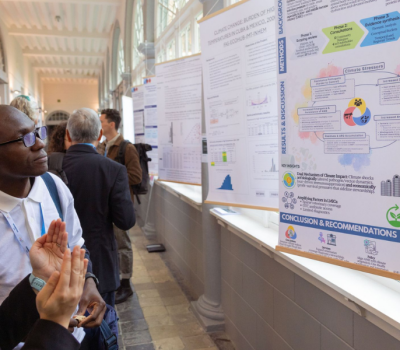
As part of work package 2 in the SECRET project - improving clinical trial review and oversight - a training workshop for the TASO Research Ethics Committee (REC) was held in Kampala, Uganda from 29 to 31 July 2025.
The TASO REC is hosted at the AIDS Support Organization (TASO) in Uganda. This training followed a previous needs assessment, which identified specific knowledge gaps in reviewing and monitoring clinical trials. In response, a tailored training manual was developed and used to guide the sessions. Staff of the clinical trials unit of the National Drug Authority (NDA) also took part.

The workshop brought together 12 TASO REC members and 7 NDA staff for the three-day programme. The training was held under the theme: “Review and Monitoring Clinical Trials” and covered key topics such as:
Clinical Trial Designs
Ethical Review of Randomized Controlled Trials (RCTs)
REC Oversight of Investigational Product Accountability in RCTs
Risk–Benefit Assessment in Clinical Trials
The trainers included SECRET project personnel, as well as experts from Makerere University, NDA, Mulago National Referral Hospital, Uganda National Health Research Organisation (UNHRO) and Uganda National Council for Science and Technology (UNCST).

Before this training, the TASO REC had not reviewed any clinical trial protocols. Participants of the workshop expressed appreciation for the training, highlighting that it enhanced their knowledge and skills in reviewing and providing oversight for clinical trials. They are now eager to receive RCT protocols.
They also felt that other RECs in Uganda needed similar capacity-building efforts. To measure the impact of the training, both a pre-test and post-test was conducted. The results showed a significant improvement in individual test scores. Each participant received a certificate of attendance at the end of the workshop.
The training not only strengthened the capacity of TASO REC and NDA staff but also reinforced the commitment to ensuring ethical and scientifically sound oversight of clinical research in Uganda.
Spread the word! Share this story on