Why 200,000 children still die every year due to antibiotic resistance

In Europe, antibiotic resistance mainly affects the elderly and hospital patients, while in low-income countries it is mainly children who die from infections that are resistant to antibiotics. In Burkina Faso, scientists are investigating how people come into contact with these bacteria at home and in their immediate environment, and how great the risk of becoming ill is.
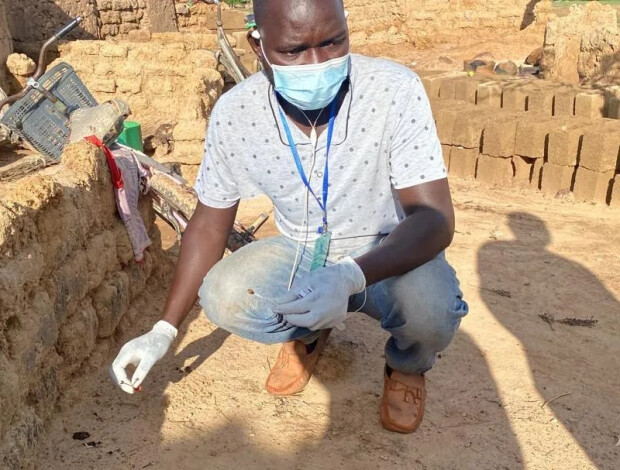
Daniel uses some fishing line to fish a full cup of toilet contents out of the latrine shared by several families. He then filters a litre of water from a barrel of drinking water stored by the family. He looks for a sample of chicken droppings around the house. Finally, he obtains samples of three family members' morning bowel movements, packed in matchboxes. Daniel immediately continues his journey to twelve other families in the village, while his colleague Stéphane spends three hours observing how often two-year-old Ousmane puts his hand on the ground or in his mouth.
At the hospital in Nanoro, where Daniel and Stéphane have their research centre, in central Burkina Faso, it is mainly children who die from infections with resistant bacteria. The children are only brought to hospital at a late stage of their illness. They have usually already taken malaria medication and antibiotics, administered by a nurse at a health centre, without effect. Once in the hospital, it is often unclear how to treat the infection. A test to identify the pathogen and determine the appropriate course of antibiotics can take up to seven days.
The resistant bacteria that cause these infections are in most cases transmitted outside the hospital and end up in the intestines, throat, lungs or skin. They remain there for a long time, until the host becomes weakened by malaria, a viral infection or malnutrition. Then the bacteria seize their opportunity to infect an organ or even the entire body. Recently completed research by the two Burkinabe researchers revealed that two out of three healthy family members are carriers of an intestinal bacterium that cannot be treated in the local hospital when it enters the bloodstream. In Europe, only one in twenty people are carriers of such an untreatable intestinal bacterium.
In Europe, the fight against antibiotic resistance focuses primarily on improving antibiotic use and preventing hospital infections. In Burkina Faso and many other low-income countries, the solution must consist of both intercepting the resistant bacteria before they reach the intestines or lungs and keeping the host healthy. Much progress has been made in the latter area in recent decades. Vaccination rates have more than doubled since 2000. Malaria infections and malnutrition have almost halved, and a new malaria vaccine offers additional hope. Most births now take place under the supervision of a midwife, compared to less than one in three in 2000.
However, steps still need to be taken to prevent the spread of resistant bacteria in and around the home. Measuring where bacteria are present, the extent to which each family member is exposed to them, and whether those bacteria actually end up in the intestines or lungs should help in deciding which approach is most effective: improving water availability or water quality, building toilets, or improving hand hygiene.
"We want to predict effective antibiotic treatments based on environmental samples, thereby saving valuable time."
Back in the microbiology laboratory, right next to the Nanoro hospital, Daniel now gets to work with the fresh and refrigerated stool and environmental samples. He applies small amounts of the various samples, such as stool, soil, food, water and chicken droppings, to a culture plate using a cotton swab. This is a 15 cm wide dish containing a gel with an antibiotic, which means that only resistant bacteria will grow on it. After keeping the culture plate at 37°C for 24 hours, Daniel checks for the first time to see if bacterial colonies are growing, visible as coloured dots.
After several cycles, Daniel will extract DNA from each bacterial colony. Researchers will use this DNA to map the entire genome of the bacterium, including all the genes that cause resistance. The presence of the same genes in bacteria from the family's environment, in bacteria found in the faecal samples of family members, and in the blood of seriously ill children, allows us to estimate the contribution of each environmental source to disease burden and mortality. Dutch researchers used the same technique to discover that two-thirds of Listeria infections, which can lead to premature birth, blood poisoning or meningitis, were caused by eating raw beef or soft cheeses. The remaining third came partly from chicken meat and smoked salmon.
Genome analyses of potential sources of infection in and around the home may be useful for other purposes. In most hospitals in low-income countries, patients with serious infections are not routinely tested for bacterial infections because testing infrastructure is not available or is too expensive, and because results are only available five to seven days after blood sampling – often too late for seriously ill patients. Researchers have already predicted which antibiotics are needed to effectively treat resistant bacterial infections in a hospital based on the resistance genes present in the stool of patients in that hospital.
Now, the team in Nanoro, together with researchers at the Institute of Tropical Medicine in Antwerp, mathematicians at the University of Oxford, and bioinformaticians at the University of Basel, want to go one step further and predict effective antibiotic treatments based on environmental samples. We are evaluating and comparing which environmental samples or combinations of samples, from different environmental sources or different villages, provide the most reliable predictions. With a reliable prediction, doctors would be able to start the most effective antibiotic treatment immediately, rather than having to wait five days for test results.
After spending the morning visiting families by motorbike in 35-degree heat and the afternoon in the laboratory at 18 degrees, Daniel and Stéphane each write a scientific article in the evening. Burkina Faso is going through a difficult period. For ten years, a series of attacks and assaults by various Islamic extremist groups have destabilised the country. Large parts of the country are no longer under government control. Deadly attacks, including on schools, continue. Throughout all these years, Daniel and Stéphane's research group has continued to conduct research.
The group led clinical trials of two new malaria vaccines. Both vaccines are now recommended by the World Health Organisation – a major boost to the global fight against malaria. Daniel remains optimistic that the government, now under military rule after two coups in 2022, will be able to restore stability within a short period of time. This would allow research into antibiotic resistance to be translated into concrete improvements in access to clean water and toilets, fewer resistant bacterial infections, and a further decline in child mortality.
This article was translated from Dutch and originally published by EOS Wetenschap.


Brecht Ingelbeen
Dr Brecht Ingelbeen is an infectious disease epidemiologist and researcher at the Institute of Tropical Medicine in Antwerp. He uses population-based studies to investigate factors that contribute to antibiotic resistance worldwide and how it can be most effectively combated. His research is supported by the Research Foundation – Flanders. He previously worked for Médecins Sans Frontières, the French Public Health Agency, and the International Centre for Antibiotic Resistance Solutions.
Spread the word! Share this story on