Towards ending the scourge of anaemia in pregnancy in Nigeria
Globally, about 1 in 3 pregnant women suffer from anaemia, a condition in which the blood does not have enough healthy red blood cells. It is most common in regions where there is low literacy rate, high levels of poverty and malnutrition. Most affected regions of the world are countries within sub-Saharan Africa and Southeast Asia. Recent research called the IVON TRIAL has found a potential solution to combat some of the problems associated with treatment of anaemia in low- and middle-income countries. This will help in facilitating the attainment of Sustainable Development Goal 5 as it relates to maternal (mother) and perinatal (child) health. The IVON TRIAL was a collaboration between ten institutions from Nigeria, Sweden, the UK, and the US and Belgium, and was funded by the Bill and Melinda Gates Foundation. Aside research, the IVON TRIAL helped to build capacity in Nigeria by training two doctoral students, who here share their experiences.
Read journal article
The article about the IVON trial appeared in The Lancet Global Health on 18 September 2024.

The two doctoral students are Ochuwa Babah and Opeyemi Akinajo, both obstetricians and researchers in Nigeria with keen interest in maternal and foetal health. “It was the largest clinical trial I have ever been involved in,“ said Dr Ochuwa. “Even though I was a co-investigator in another clinical trial before this, the IVON TRIAL gave me an opportunity to acquire vast experience on the design and conduct of large clinical trials. It opened my eyes to the numerous challenges one can face during this type of work.” Her colleague, Dr Opeyemi says: “Implementation science was very new to me. Initially I did not know how to go about it, but eventually I learnt, and now it is all turning out well.”

The studies
The IVON TRIAL birthed many studies. The studies were conducted in Lagos and Kano State, the two most densely populated states in Nigeria with significant differences in the sociodemographic and cultural attributes of the women. It looked into who, where, why, how, and what regarding anaemia in pregnancy in Nigeria. According to Dr Ochuwa, “in the first study, we found that 4 out of every 10 pregnant women in Nigeria who had anaemia of moderate or worst severity, had iron deficiency. We also found that diet played a role; consuming soybeans three to four times a week or eating edible kaolin clay almost every day predisposes to a higher risk of having iron deficiency anaemia, whereas eating green leafy vegetables reduces the risk for iron deficiency anaemia by over 60%. Now, we have a better idea how to counsel our pregnant women and advise them on things to avoid.”

To corroborate the IVON TRIAL was a large survey on maternal healthcare workers in 84 public health facilities conducted by Dr Ochuwa and team, to understand how the healthcare workers screen and treat pregnant women for anaemia in pregnancy in Nigeria. “Most of the healthcare workers do not screen women routinely for iron deficiency before giving iron because of the cost of the tests and lack of laboratory facility in some places. 96% of the healthcare workers treat their patients with iron tablets. Intravenous iron is perceived to be expensive.” This was supported by the findings of Dr Opeyemi when she explored the acceptability of using intravenous iron for anaemia treatment in Nigeria. Opeyemi added “Pregnant women and their families are willing to accept intravenous iron. The healthcare workers are willing to prescribe and administer it if they are trained on how to use it, if constant supply of the medicine is guaranteed, and the cost of the medicine is subsidized.”
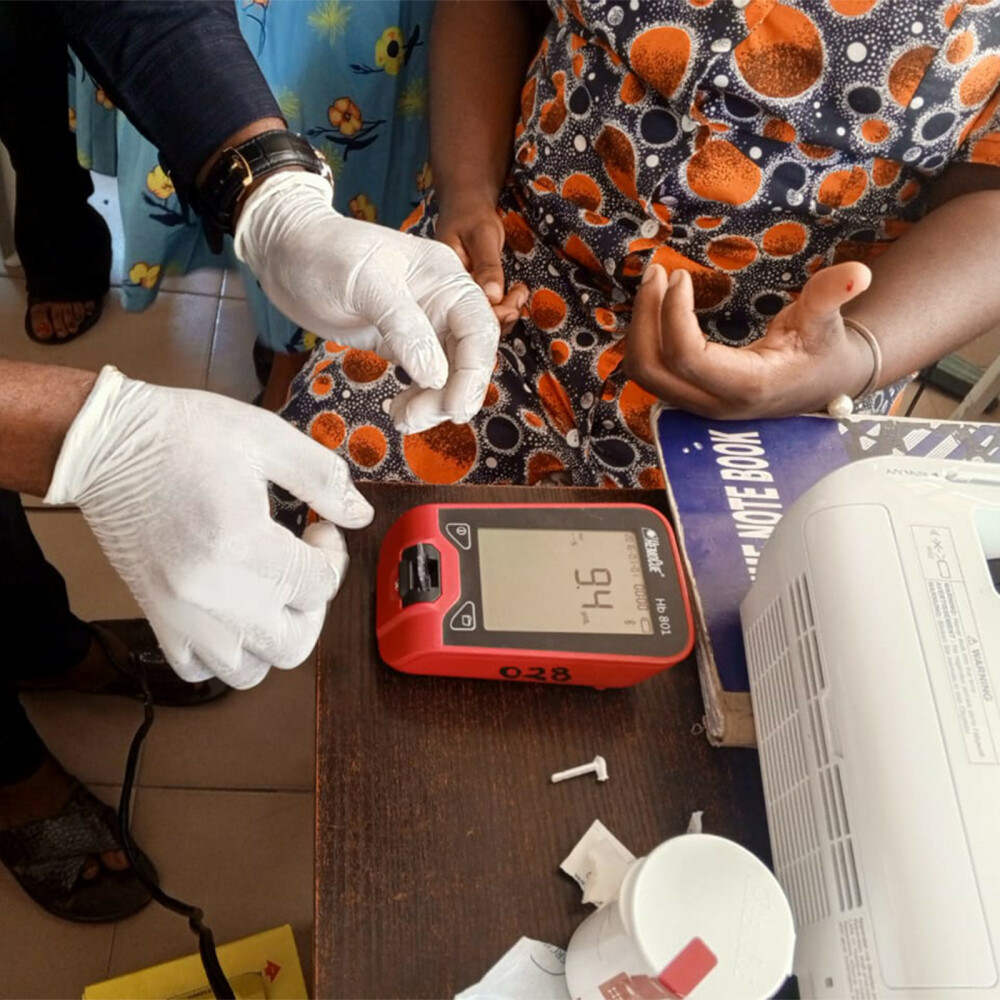
The IVON TRIAL found that taking only one dose of a new intravenous medication called “ferric carboxymaltose” during pregnancy worked faster and better than taking iron tablets three times every day till delivery. For anaemic women who lacked iron, as delivery draws near, giving iron through the vein reduces the risk of being iron deficient or of having iron deficiency anaemia by over 70%. The intravenous iron is as safe as the tablet. Most exciting is that a woman only needs to spend an additional 45-60 minutes in the hospital without formal admission to receive the intravenous iron. Dr Ochuwa says: “the common reasons why some pregnant women remain anaemic despite treatment is because they often forget to take the tablets, or do not tolerate them because of side effects like nausea, vomiting or diarrhoea or they don’t just like the smell or taste of it. Now we have identified an effective and safe alternative and can help our pregnant women better.” Every year many women die as a result of anaemia or its complications, as it can predispose a pregnant woman to a higher risk of having a preterm birth or of excessive bleeding at or soon after childbirth, or even to heart failure. In addition, the baby of an affected woman may fail to achieve the full growth or die in the womb. “Ferric carboxymaltose is new to us here in Nigeria because it was never available before this research, and we know it is the same situation in most parts of sub-Saharan Africa. Now we have better knowledge to help pregnant patients.”
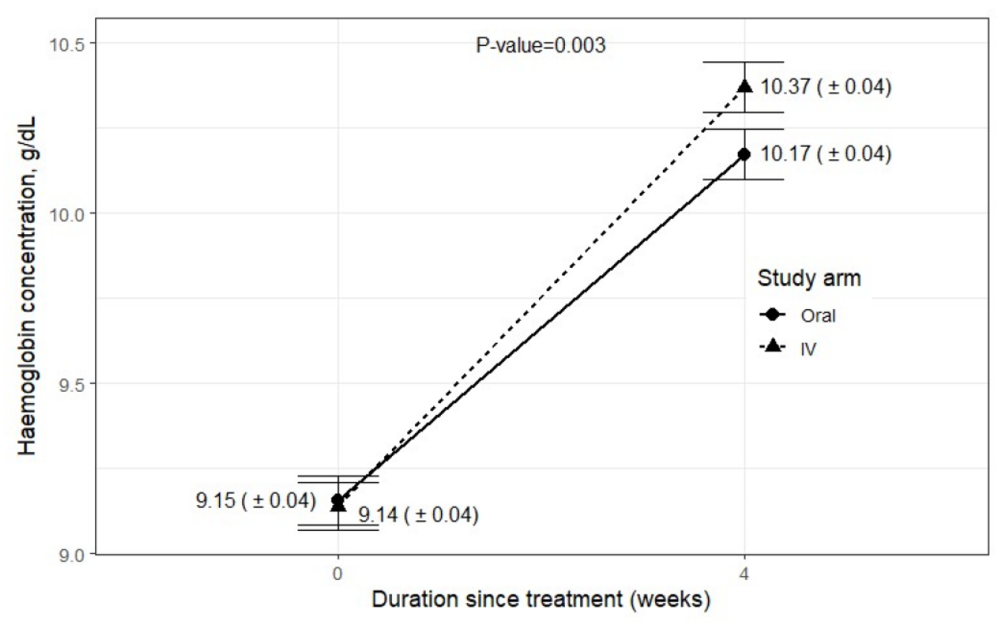
Dr Opeyemi assessed the capability of our maternal healthcare workers to administer the intravenous iron. She says, “overall, the maternal healthcare workers were able to administer the medicine correctly according to laid down protocol 66% of the times. We observed their performance in this regard was highest in the tertiary health facilities and poorest in the primary health facilities. We found that the low score at some health facilities is due to shortage of staff and large patient load which makes it difficult for the healthcare workers to be focused. And for facilities where it was most successful, we observed that they displayed a good teamwork. Now, we know the barriers and the most affected facilities, we know who and where to target before implementing the use of this new medicine.”

Challenges of conducting clinical trials
“Nothing in life goes without a challenge” says Dr Ochuwa. “The IVON TRIAL was an eye-opener to many challenges associated with clinical trials in low-middle-income countries. During the clinical trial, I was able to understand the dynamics involved, the interplay between research and culture, transcultural barriers to research, and ways to overcome these challenges. We identified the need for male partner involvement in research into women’s health. We identified fear of potential harm as a major problem, especially for research on pregnant women where two lives are involved. Proper counselling did the trick in the IVON TRIAL. People need to understand that it is only through research we can achieve scientific progress.”
Reflections on doctoral training
Ochuwa and Opeyemi are doctoral students at the Department of Global Public Health, Karolinska Institutet, Sweden, and also fortunate to be light PhD students at Institute of Tropical Medicine, Belgium; both funded from the IVON TRIAL grant. Dr Ochuwa says “My doctoral training has expanded my knowledgebase in research and taught me how to do research correctly. It expanded my skills in clinical trials, data analysis, project management, and presentation of research findings to large and diverse audience in difference ways. And it has increased my network.” According to Opeyemi, “The journey has been challenging and inspiring, pushing me to integrate my academic pursuits with my professional and personal responsibilities. However, with my supervisors' unwavering support and guidance, I have honed my research skills, expanded my horizons, and they equipped me with resilience and optimism, fueling my passion for research and academic growth.” A word of advice to upcoming doctoral students from Dr Ochuwa: “You need to be committed, persistent, persevere, have the ability to withstand stress and be willing to learn new skills to achieve a successful doctoral education. Talk to colleagues, ask questions, no one knows it all, this was my approach. Your supervisors are there to guide you, but they are not to do it for you; they are training you to be an independent researcher and this you must achieve to bag your doctoral degree. ”.
The researchers believe that their work can have impact on the global community. “We strongly believe that the knowledge gained so far will help us and our medical colleagues modify our practice to give the best of care to our women. This will help us reduce illnesses and deaths of mother and babies. We have started spreading the news about our findings through journal publications, at conferences and seminars. The Principal Investigator, Professor Bosede Afolabi is also liaising with the Federal Ministry of Health, Nigeria to include intravenous ferric carboxymaltose on the essential drug list,” concludes Dr Ochuwa.
Spread the word! Share this story on