The last mile of sleeping sickness elimination


A declining but persistent threat
Human African trypanosomiasis (HAT), commonly known as sleeping sickness, is a deadly vector-borne parasitic disease that has plagued sub-Saharan Africa, particularly the Democratic Republic of the Congo (DRC), for decades. Transmitted through the bite of an infected tsetse fly, the disease progresses silently, with symptoms often mistaken for more common ailments in its early stages. Without treatment, sleeping sickness is almost always fatal.
The disease was once widespread, but has been dramatically reduced in recent years thanks to rigorous control efforts. The World Health Organization (WHO) has set the ambitious goal of interrupting the transmission of sleeping sickness by 2030—a milestone that once seemed within reach. Yet, ITM experts warn that without continuous vigilance, screening and community participation, sleeping sickness could resurge, despite years of progress. "In a world plagued by other massive health challenges, 800 to 900 cases a year might seem trivial," says ITM parasitologist Jakke Van den Abbeele. "But if we don’t sustain the effort, the disease will seize its chance and, in 10 to 15 years, it could resurge."

The promise of acoziborole in treatment
Two subspecies of HAT can be distinguished: Trypanosoma brucei gambiense (gHAT), which is found in West and Central Africa and is responsible for 92% of reported cases, and Trypanosoma brucei rhodesiense, which occurs mainly in East and Southern Africa.
Until recently, treating gHAT was a complicated and often risky process. The available treatments were toxic and required a multi-step diagnostic process: serological screening, microscopic confirmation, and a lumbar puncture to determine the disease stage. This cumbersome approach led to the loss of up to 50% of cases, as many patients never completed the full process. The situation was further complicated by limited healthcare infrastructure in rural areas, making follow-ups difficult. Many individuals also avoided testing due to the fear of invasive procedures.
The landscape changed dramatically with the development of acoziborole, a single-dose oral treatment that has the potential to transform how sleeping sickness is managed. Recent phase III clinical trials showed that acoziborole is 98.1% effective, regardless of disease stage. More importantly, it eliminates the need for lumbar punctures, making it safer, simpler, and more accessible.
With this breakthrough, experts envision a screen and treat approach, where individuals who test positive can receive immediate treatment—eliminating the need for complex confirmatory procedures and increasing the chances of curing patients before they develop severe symptoms.

StrogHAT brings acoziborole to the field
The EDCTP-funded StrogHAT project is at the forefront of implementing this new treatment strategy in real-world settings. The project’s mission is clear: to provide the first evidence needed to integrate acoziborole into national HAT control programmes. “The treatment has proven to be surprisingly effective,” declares Elena Nicco, infectious disease specialist and Principal Investigator of the StrogHAT project at ITM. “Three pills, and then you’re treated. However, treating an entire population of 300 million for a disease affecting a couple of hundred people isn't feasible. Instead, the focus must remain on targeted interventions, continued screening, and robust funding.”
By simplifying diagnosis and treatment, StrogHAT aims to increase patient acceptance, improve access to care, and ultimately accelerate the elimination of gHAT, supporting WHO’s goal of stopping transmission by 2030.
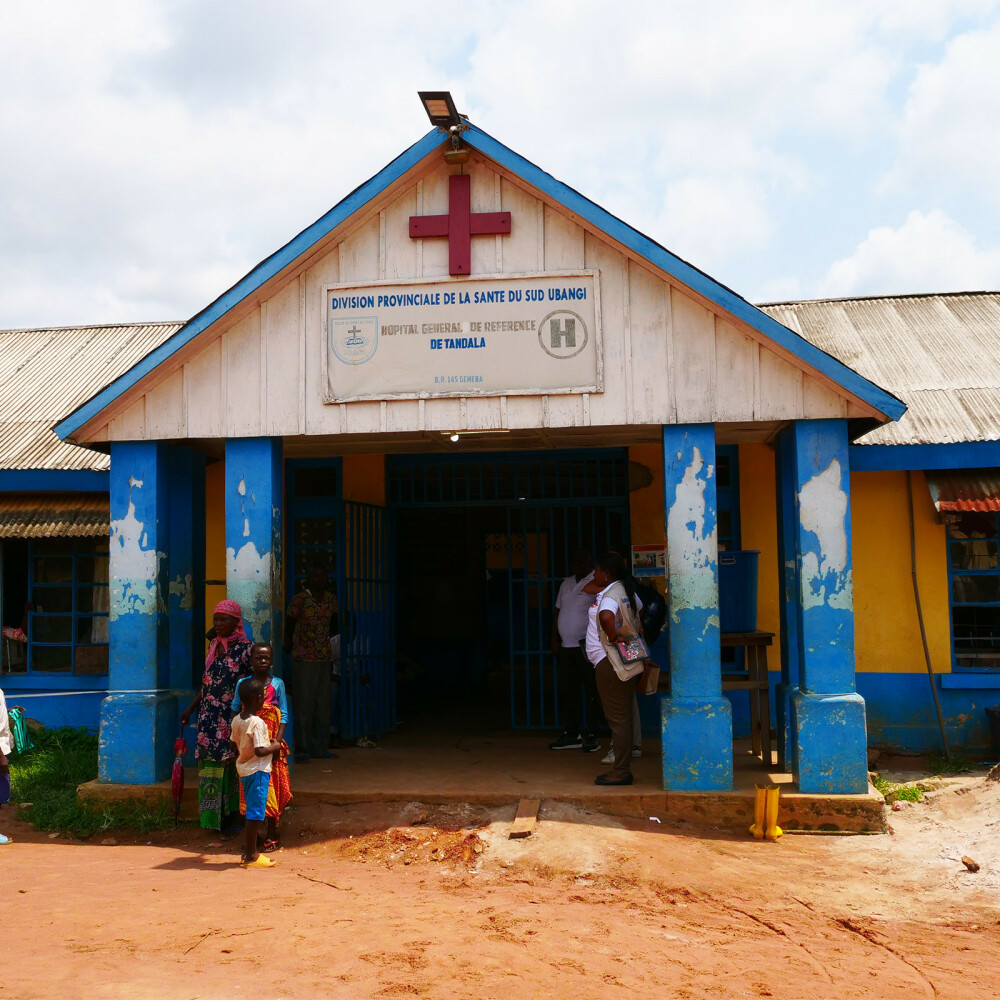
First patients receive treatment
September 2024 marked a significant moment within the StrogHAT project, as the first patients were enrolled in the clinical trial. On September 15, the first subject received acoziborole. Since then, over 100 seropositive gHAT patients have been treated. The study is ongoing, with new cases identified through mass screenings in endemic villages and passive case detection across 31 health facilities in the Equateur Nord region of the DRC.
The dedicated teams behind StrogHAT continue their efforts, with preparations underway for the next phase of monitoring. Their commitment brings hope that sleeping sickness will soon be a disease of the past, ensuring that years of progress are not undone. With sustained support and innovative treatments like acoziborole, a future without sleeping sickness is closer than ever.
The StrogHAT project is funded by the European Union’s Horizon Europe research and innovation programme, Global Health EDCTP3, and led by ITM, in collaboration with Institut National de Recherche Biomédicale (DRC), Programme National de Lutte contre Trypanosomiase Humaine Africaine (DRC), Institut de Recherche pour le Développement (France), and Drugs for Neglected Diseases Initiative (Switzerland).
(c) Header: Veerle Lejon ( Institut de Recherche pour le Développement)

Want to learn more about Jakke and Elena's work?
They will share their personal experiences and insights from the last mile of sleeping sickness elimination in the third season of our ITM podcast Transmission—coming March 2025!
Spread the word! Share this story on